Figure 5.
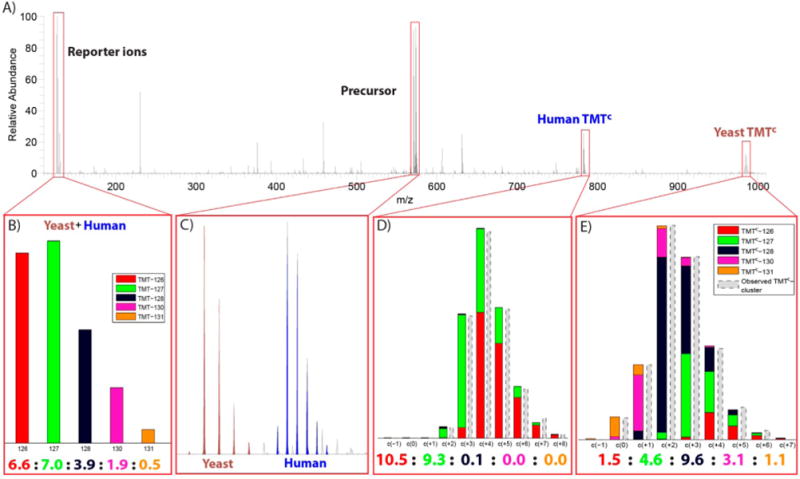
TMTC ion clusters allow quantification of multiple peptides from a single MS2 spectrum. (A) MS2 spectrum from an analysis of the two-proteome human–yeast sample applying a ±3 m/z isolation window. (B) The ratios of the reporter ions (normalized to 20) indicate that peptide ions of both human and yeast origin were fragmented in this MS2 experiment. (C) Intact precursor ions of a doubly charged (brown) and triply charged (blue) peptide ions were detected. The peptide ions were identified as YTTLGK from yeast (+2) and LDEREAGITEK from the human sample (+3). (D) The contribution of each TMT channel (colored) to the TMTC ion cluster of the human peptide is determined through deconvolution. (E) Equivalent representation of the TMTC ion cluster from the yeast peptide. For both panels D and E the ratio predictions based on deconvolution are close to the actual mixing ratios of the yeast and human peptides in the two-proteome sample.
