Fig. 2. The rotavirus replication cycle.
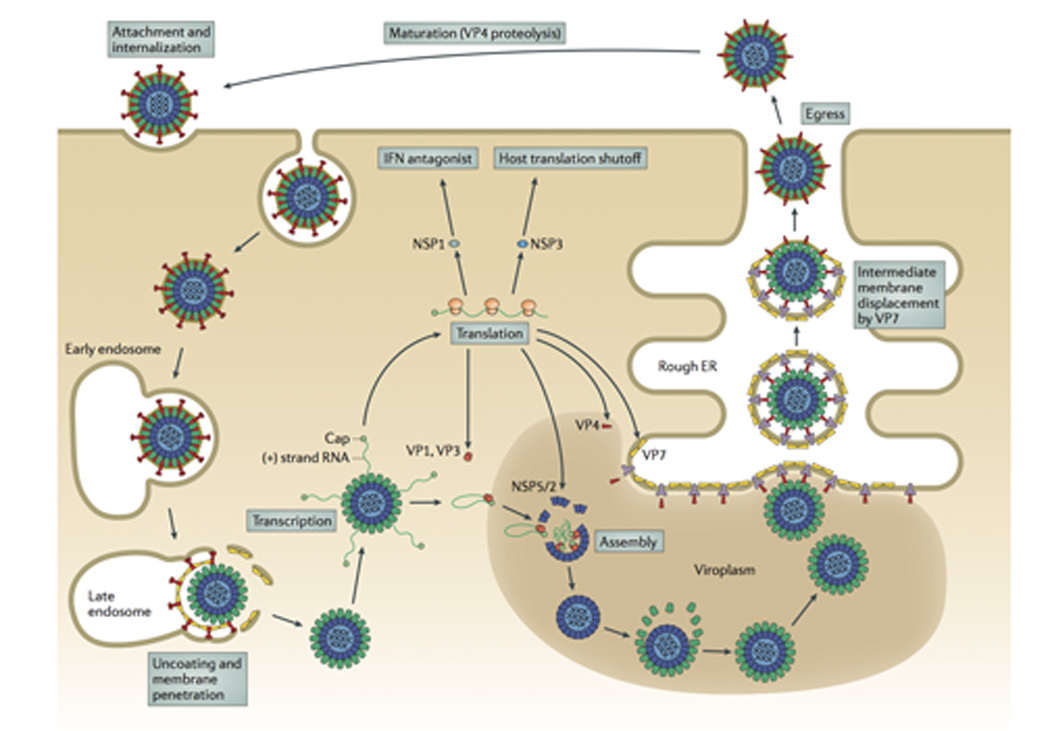
The rotavirus virion first attaches to the target cell; many strains bind cell-surface sialic acids through VP8* at the tips of the virion spikes (red). Non-clathrin, non-caveolin mediated endocytosis delivers the virion to the early endosome. There, reduced calcium concentrations are thought to trigger uncoating of VP7 (yellow) and membrane penetration by VP5* (red). Loss of the outer capsid and release of the DLP into the cytosol activates the internal polymerase complex (VP1 and VP3) to transcribe capped (+)RNAs (grey) from each of the eleven dsRNA genome segments. (+)RNAs serve either as mRNAs for viral protein synthesis by cellular ribosomes or as templates for (-)RNA synthesis during genome replication. Two non-structural proteins, NSP2 and NSP5, interact to form large inclusions (viroplasms; brown) that sequester components required for genome replication and sub-viral particle assembly. Genome packaging is initiated when VP1 (and, presumably, VP3) complex with the 3’ end of viral (+)RNAs. It is currently thought that interactions among the eleven (+)RNAs drive formation of the “assortment complex”. Condensation of the inner capsid protein, VP2 (light blue), around the assortment complex triggers dsRNA synthesis by VP1. The intermediate capsid protein, VP6 (green), then assembles onto the nascent core to form the DLP. Assembly of the outer capsid is not well understood; the current model proposes that interaction with the viral transmembrane protein NSP4 (dark blue) recruits DLPs and the outer capsid protein VP4 (red) to the cytosolic face of the endoplasmic reticulum (ER) membrane. Through an undefined mechanism, the DLP/VP4/NSP4 complex buds into the ER. Subsequent removal of the ER membrane and NSP4 permits assembly of the ER-resident outer capsid protein VP7 and formation of the triple-layered virion (see Fig. 6). Release from the infected cell exposes the virion to trypsin-like proteases of the gastrointestinal tract, resulting in the specific cleavage of VP4 (wiggly red) into VP5* and VP8* (straight red) to produce the fully infectious virion.
