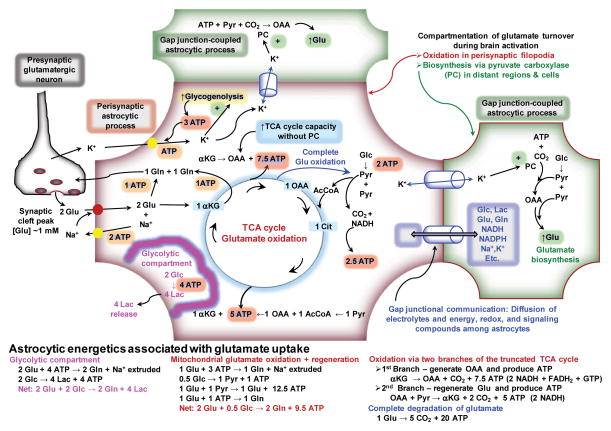
Figure 1. Energetics of astrocytic glutamate uptake.
K+ released from neurons is taken up by astrocytes and fueled by glycogenolysis. Glutamate (Glu) uptake into perisynaptic processes of astrocytes that contain mitochondria has two fates, conversion to glutamine (Gln) or oxidation. When partially oxidized, the glutamate is converted to α-ketoglutarate (αKG), then oxaloacetate (OAA). This OAA can be converted to aspartate (not shown) and it can serve to increase the catalytic capacity of the TCA cycle without increased pyruvate (Pyr) carboxylase (PC) activity. Glu can be regenerated after condensation of OAA with acetyl CoA (AcCoA) and passage through the TCA cycle. Alternatively, Glu can be completely oxidized by exit of malate from the TCA cycle (blue arrow from TCA cycle to Pyr), its conversion to pyruvate in the cytoplasm, and reentry of pyruvate into the TCA cycle (See text). Energy derived from glutamate oxidation is obtained from its shunting through the TCA cycle and by complete oxidative degradation. Because electrolytes, metabolites, and signaling compounds can diffuse within the astrocytic syncytium via gap junctions, local K+ concentrations may increase in regions of the astrocyte distant from the perivascular zone or in gap junction-coupled astrocytes, stimulate pyruvate carboxylase and increase de novo glutamate synthesis. Compartmentation of glutamate oxidation in perisynaptic regions and glutamate synthesis in more distant structures is denoted by the red and green borders, respectively. A glycolytic compartment is included for structures that do not contain mitochondria.