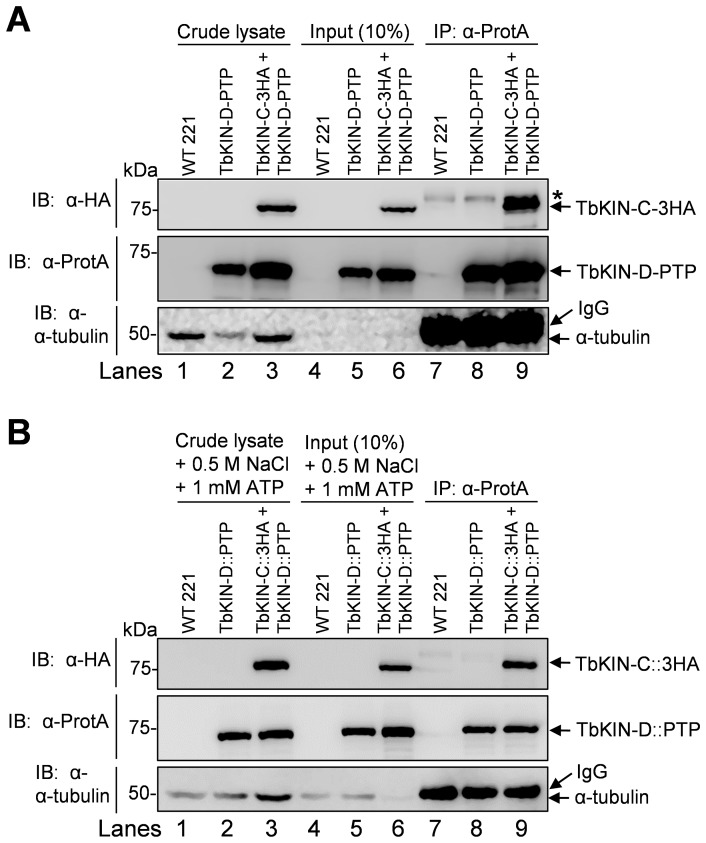
Figure 1. TbKIN-C and TbKIN-D form a complex in vivo in the bloodstream form.
(A). Co-immunoprecipitation to detect the in vivo interaction between TbKIN-C and TbKIN-D in the bloodstream form of T. brucei. TbKIN-D-PTP and TbKIN-C-3HA were co-expressed from their respective endogenous locus in wild-type 221 cell line. Co-immunoprecipitation was carried out by incubating the cleared cell lysate with IgG sepharose beads and subsequent immunoblotting of immunoprecipitates with anti-HA antibody, anti-Protein A antibody, and anti-tubulin antibody, respectively. The asterisk indicates a non-specific band recognized by the anti-HA antibody. (B). The in vivo interaction between TbKIN-C and TbKIN-D is not bridged by microtubules. Crude cell lysates were incubated with 1 mM ATP and 500 mM NaCl to dissociate kinesin proteins from microtubules, and then cleared by centrifugation before immunoprecipitation. Co-immunoprecipitation was carried out as in (A). Lanes 1–3: crude cell lysate: boiled cell lysate without centrifugation; Lanes 4–6: Input: centrifugation-cleared cell lysate that was used for immunoprecipitation; Lanes 7–9: immunoprecipitation. The higher molecular mass band detected by anti-tubulin antibody in lanes 7–9 was the IgG heavy chain, which is slightly bigger than tubulins (50 kDa).