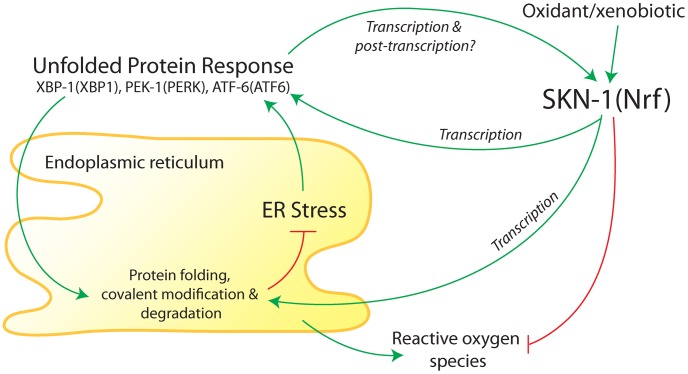
Figure 1. Summary of interactions between the endoplasmic reticulum (ER) unfolded protein response (UPR) and antioxidant/detoxification transcription factor SKN-1 in C. elegans.
The ER folds and modifies newly synthesized membrane and secreted proteins. Accumulation of misfolded proteins in the ER activates three canonical branches of the UPR, which are mediated by XBP-1, PEK-1, and ATF-6 (mammalian homologs in parentheses). Transcriptional targets of the UPR function to promote protein folding and covalent modification of new proteins and degradation of misfolded proteins. SKN-1 (homolog of mammalian proteins Nrf1, Nrf2, and Nrf3) was found to transcriptionally regulate core UPR regulators and some downstream targets during ER stress [1]. Core UPR regulators also transcriptionally regulate SKN-1 during ER stress and oxidative/xenobiotic stress [1]. Based on studies in mammalian cells [12], [13], SKN-1 would also be expected to buffer reactive oxygen species that are produced in the ER. A long variant of SKN-1 may reside in the ER membrane (not shown). Regulation of SKN-1 during ER and oxidative/xenobiotic stress may include post-translational modifications.