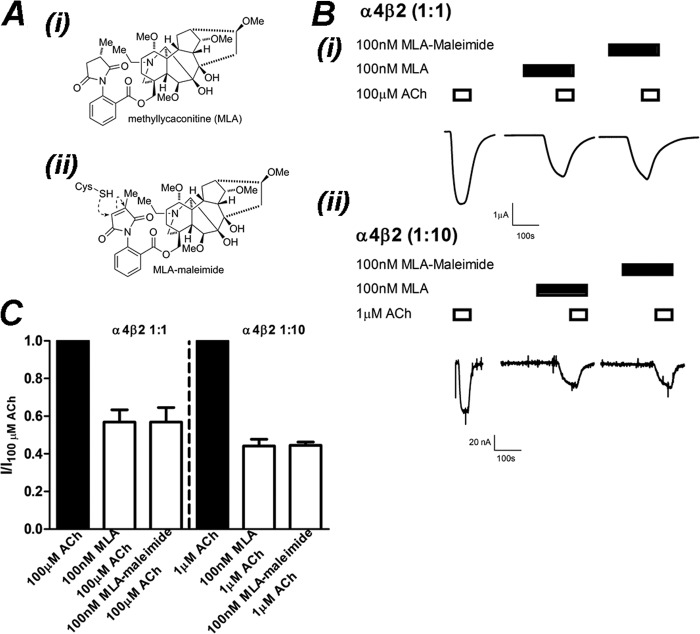
FIGURE 6.
MLA-maleimide inhibition of α4β2 nAChRs is identical to MLA. A, the chemical structure of MLA (i) and MLA-maleimide (ii) shows the location of the reactive maleimide group. B, shown are raw traces from a single experiment demonstrating inhibition of ACh-activation of (α4)3(β2)2 (i) and (α4)2(β2)3 (ii) receptors by 100 nm MLA or 100 nm MLA-maleimide after a 3-min incubation. C, shown is the mean ± S.E. of current responses normalized to 100 or 1 μm ACh for (α4)3(β2)2 and (α4)2(β2)3 nAChRs, respectively. Inhibition of ACh (filled bars) by 100 nm MLA or 100 nm MLA-maleimide (open bars) after 3 min of incubation was not significantly different at (α4)3(β2)2 or (α4)2(β2)3 nAChRs (p > 0.05; Student's t test; respectively, n = 4).