FIGURE 5.
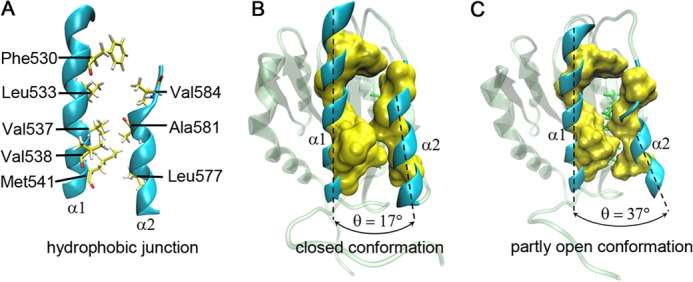
The representative closed and open conformations of the hydrophobic core in A1 domain. A, the zipper-like hydrophobic junction between the α1- and α2-helices in Newcartoon representation. All involved hydrophobic residues (Phe530, Leu533, Val537, Val538, Met541, Leu577, Ala581, and Val584), functioned as the “zipper teeth,” in the α1- or α2-helix are represented as yellow licorices. B, a closed state of the hydrophobic core in the mutant R543Q with the α2-α1 interhelical angle of 17°. C, this closed conformation becomes an open one; as such, the partly open conformation with the α2-α1 interhelical angle of 37°. The solvent-accessible surface (yellow) is displayed around the hydrophobic residues with a probe radius of 1.4 Å (B and C). The partly exposed hydrophobic residues (green licorices) (C) in the β2-strand beneath the hydrophobic junction between the α1- and α2-helices are shown in the open conformation too.
