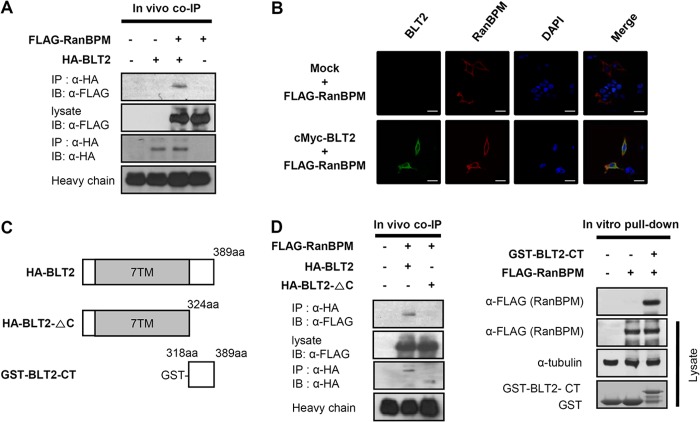
FIGURE 2.
RanBPM interacts with the BLT2 C-terminal domain. A, 3×FLAG-RanBPM and HA-BLT2 were transiently co-transfected into HEK 293T cells, and immunoprecipitation (IP) was performed using an anti-HA antibody followed by Western blotting (IB) with an anti-FLAG antibody. co-IP, co-immunoprecipitation. B, confocal images show the co-localization of RanBPM with BLT2 in the plasma membrane when the two proteins were co-expressed in HEK 293T cells. The images are pseudocolored as follows: red, 3×FLAG-RanBPM; green, c-Myc-BLT2, with yellow areas indicating co-localization in the merged image and blue indicating DAPI staining of the nucleus. Scale bars, 20 μm. C, schematic of the domain organization of BLT2. The HA-tagged deletion construct is HA-BLT2-ΔC (amino acids 1–324), and the GST-tagged deletion construct is GST-BLT2-CT (amino acids 318–389). D, 3×FLAG-RanBPM and HA-BLT2 or 3×FLAG-RanBPM and HA-BLT2 C-terminal deletion mutant were co-transfected into HEK 293T cells, and immunoprecipitation was performed using an anti-HA antibody followed by Western blotting with an anti-FLAG antibody (left). Recombinant GST or GST-BLT2-CT fusion proteins purified by glutathione-SepharoseTM 4B were incubated with 3×FLAG-RanBPM-transfected cell lysates, and the bound proteins were analyzed by immunoblotting with an anti-FLAG antibody (right). The data are representative of three independent experiments with similar results.