FIGURE 1.
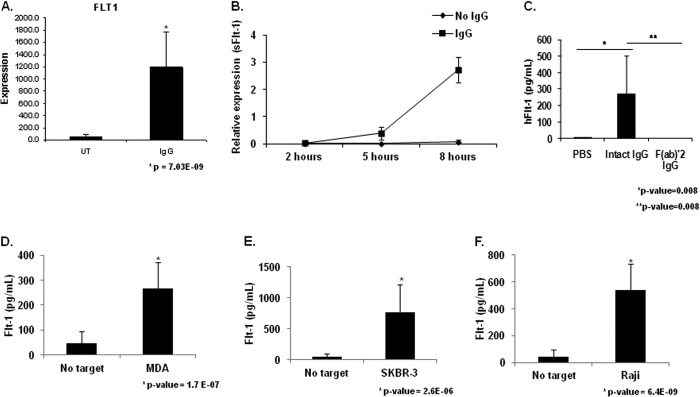
FcγR clustering by IgG leads to sFlt-1 expression by human peripheral blood monocytes. A, human PBMs were stimulated by incubation with heat-aggregated human IgG. Microarray analysis revealed increased Flt-1 expression in IgG-stimulated PBMs compared with unstimulated control (UT). B, human PBMs were incubated for the indicated time points on IgG coated wells. Quantitative real-time RT-PCR was performed with primers specific to the membrane-bound and soluble forms of Flt-1 to determine the kinetics of FcγR induction of Flt-1 expression. Significant differences in mRNA levels of sFlt-1 were observed at 5 h and 8 h relative to unstimulated controls, whereas no detectable levels of membrane bound Flt-1 were observed under any treatment condition or time point (representative donor; n ≥ 3). C, human PBMs were incubated on IgG coated plates for 24 h. Cell-free supernatants were obtained and examined for sFlt-1. No detectable levels of sFlt-1 were found in wells containing unstimulated PBMs. Whole molecule IgG is capable of inducing detectable levels of sFlt-1, whereas the F(ab)′2 portion of the IgG molecule is not (representative donor; n ≥ 3). D and F, to determine if tumor cell-bound IgG was capable of inducing sFlt-1 production after FcγR clustering, human PBMs were incubated with MDA-MB-468 (D), SKBR3 (E), or Raji (F) tumor cell lines opsonized with 10 μg/ml cetuximab, trastuzumab, or rituximab, respectively. Induction of sFlt-1 was observed for all three cell lines tested (representative donor; n ≥ 3).
