Table 3.
Pictet-Spengler cyclization of the Ugi 4-CR to yield the tricyclic 3,9-diazabicyclo[3.3.1] nonane scaffold
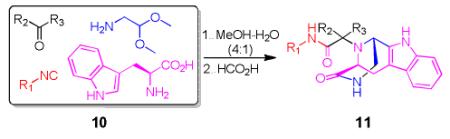
| Comp. | R1–NC |
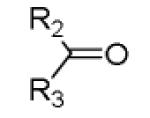
|
Yielda [%] |
|---|---|---|---|
| 11a |
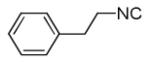
|
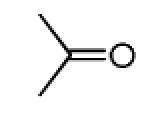
|
44 |
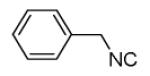
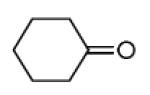
| 11b |
|
|
40 |
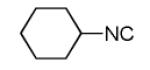
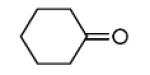
| 11c |
|
|
48 |
| 11d |
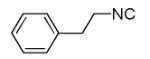
|
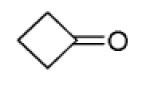
|
30 |
Overall yield in two steps in one pot (crude Ugi product was directly used for the Pictet-Spengler reaction).
