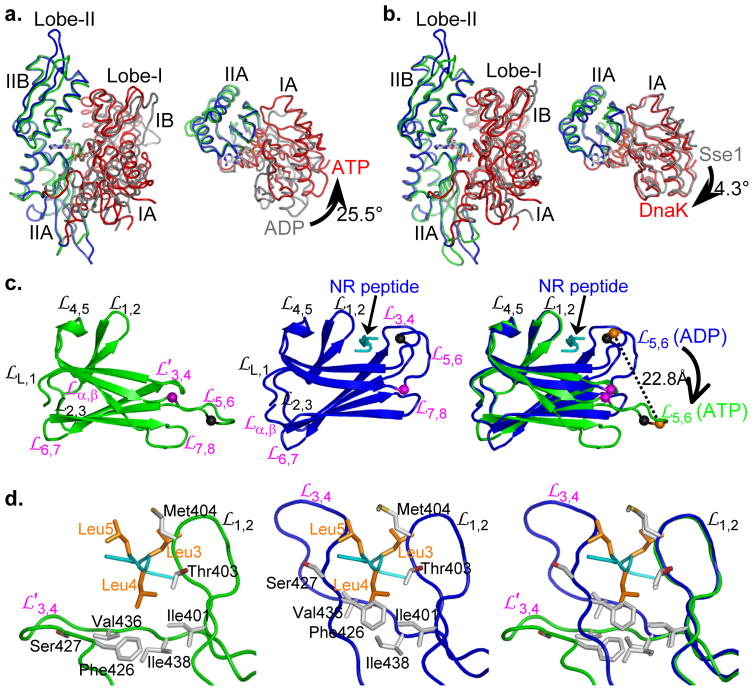
Figure 3. Unique conformations of NBD and SBD in DnaK-ATP.
a, Comparison of NBD conformations in DnaK-ATP and human Hsp70-ADP (PDB 1S3X). NBDs are superimposed based on Cαs in lobe-II. Coloring has in DnaK-ATP Lobe-I (red) and Lobe-II (blue); human Hsp70-ADP Lobe-I (grey) and Lobe-II (green). The left panel shows the canonical front-face view, and the right panel shows the view from above the left panel with IB and IIB removed to show the IA-IIA changes.
b, Comparison of DnaK-ATP and Sse1-ATP. Superpositions and coloring are as in a, except for replacement of human Hsp70-ADP by Sse1-ATP. Conformational differences between the two ATP-bound structures are slight.
c, Comparison of SBDβ conformations. The left and middle panels are ribbon diagram of SBDβs from the DnaK-ATP and the isolated DnaK-SBD (PDB 1DKZ) structures, respectively. SBDβs are superimposed based on Cαs in
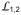 and
and
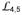 . Glycine residues Gly461 and Gly468 on loop
. Glycine residues Gly461 and Gly468 on loop
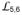 are highlighted as purple and black balls, respectively. The Cα atoms of Arg467 are highlighted as orange balls. The peptide substrate NR is in cyan. The right panel is the superposition of the left and middle panels.
are highlighted as purple and black balls, respectively. The Cα atoms of Arg467 are highlighted as orange balls. The peptide substrate NR is in cyan. The right panel is the superposition of the left and middle panels.
d, Comparison of the polypeptide-binding sites. The left and middle panels are the details of the polypeptide-binding sites in the DnaK-ATP and isolated DnaK-SBD structures, respectively. The two structures are superimposed based on Cαs in
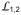 and
and
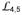 . Residues in van der Waals contacts with the three leucine residues of the NR peptide in the isolated DnaK-SBD structure are shown in sticks. The backbone of the NR peptide is in cyan, and the side chains of the three leucine residues in the NR peptide are highlighted in orange. The NR peptide in the left panel is from the isolated DnaK-SBD structure. The right panel is the superposition of the left and middle panels. The side chains of the NR peptide are not shown.
. Residues in van der Waals contacts with the three leucine residues of the NR peptide in the isolated DnaK-SBD structure are shown in sticks. The backbone of the NR peptide is in cyan, and the side chains of the three leucine residues in the NR peptide are highlighted in orange. The NR peptide in the left panel is from the isolated DnaK-SBD structure. The right panel is the superposition of the left and middle panels. The side chains of the NR peptide are not shown.