Figure 4. Two highly conserved glycine residues in
 .
.
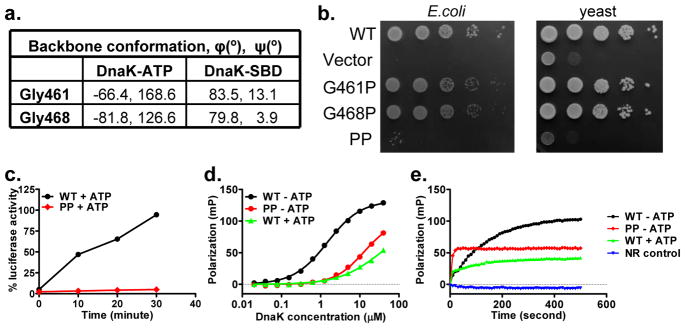
a, Comparison of phi and psi conformational angles for Gly461 and Gly468 in DnaK-ATP and isolated DnaK-SBD structures.
b, Growth tests for glycine mutants in Hsp70-deficient E. coli and yeast backgrounds. Serial dilutions of fresh cultures were spotted onto plates and incubated at 37°C. Functional DnaK and Ssa1 are required for growth at 37°C.
c, Refolding activity of WT and G461P G468P (PP) mutant DnaK. Refolding of the heat-denatured luciferase was started by adding the DnaK chaperone system (DnaK, DnaJ and GrpE) for the corresponding WT or PP DnaK.
d, Fluorescence anisotropy assay of peptide substrate binding affinity. Serial dilutions of DnaK proteins were incubated with F-NR, a model peptide substrate. Fluorescence anisotropy measurements were made after binding reached equilibrium.
e, Peptide substrate binding kinetics determined by fluorescence anisotropy assay. Immediately after mixing DnaK proteins with the F-NR peptide, peptide binding as a function of time was measured kinetics by fluorescence anisotropy.
