Figure 2.
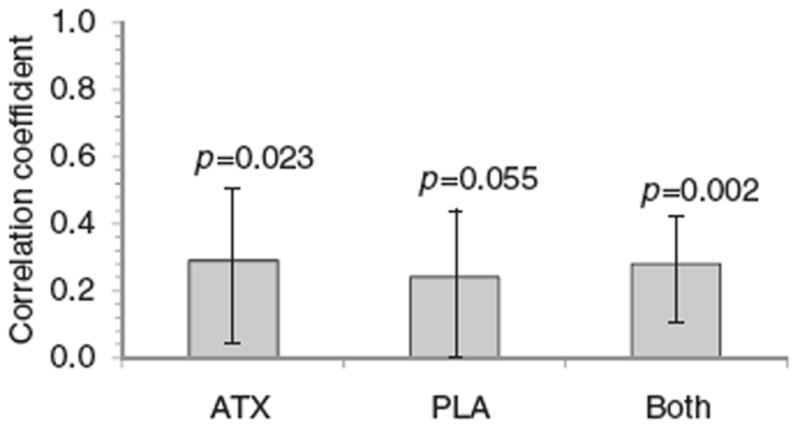
Changes in AISRS vs. changes in OCDS total scores from baseline to double-blind endpoint (or LOCF) among 61 study participants randomized to atomoxetine, and 64 randomized to placebo, who had nonmissing AISRS and OCDS data. Error bars represent 95% confidence intervals for the Pearson r correlation values. AISRS – Adult ADHD Investigator Symptom Rating Scale; ATX – atomoxetine; LOCF – last observation carried forward; OCDS – Obsessive Compulsive Drinking Scale; PLA – placebo.
