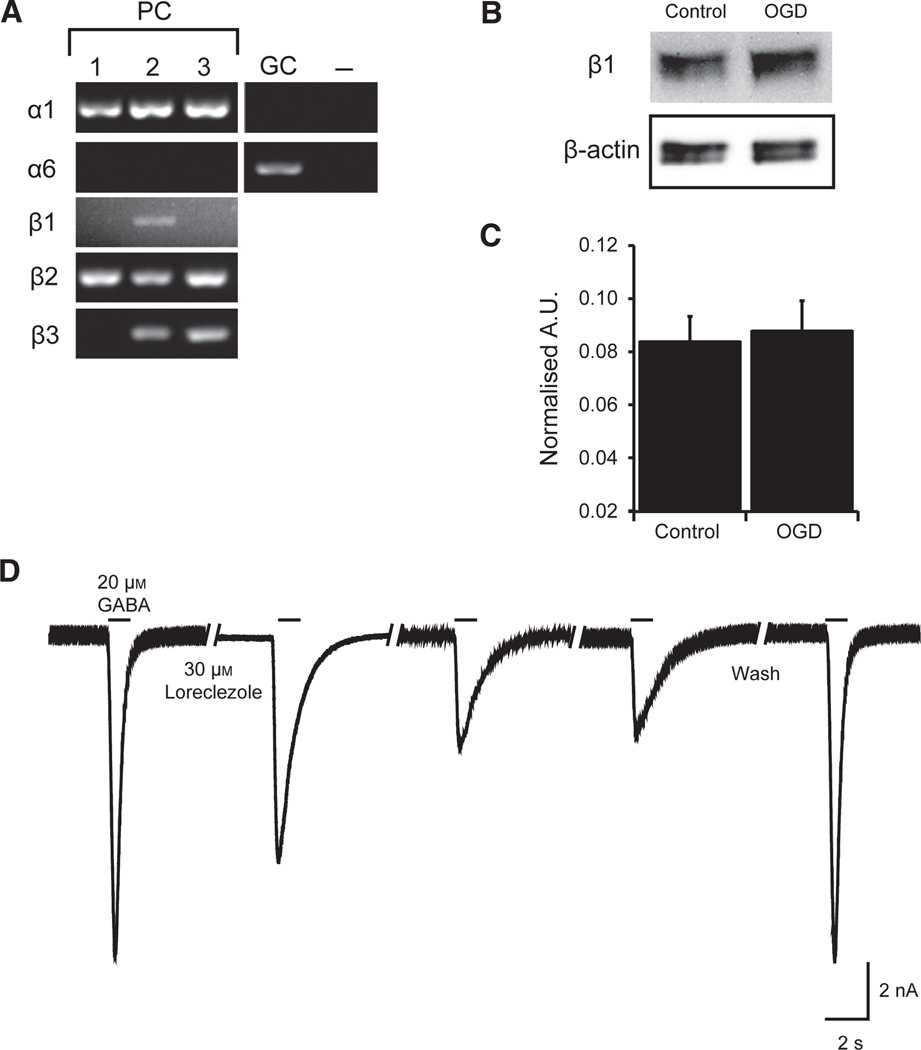
Fig. 6.
PCs express the GABAA receptor β1-subunit and protein expression of β1 is not affected by OGD. (A) Representative gels illustrating the mRNA expression of α1-, α6-β1-, β2-, and β3-subunits in isolated PCs by single-cell reverse transcription–PCR. Each lane (1–3) represents one sample containing four to six PCs. Purity of PC samples was confirmed by the absence of α6. Granule cells (GCs) were the positive control for α6. Negative control (−) was Tyrode’s solution collected from the vicinity of PCs. (B) Representative western blot of cerebellar GABAA receptor β1-subunit protein from control cultured PCs and cells that received 2 h OGD and 1 h reoxygenation. β-actin loading control shown in lower panel. (C) Quantification of GABAA receptor β1-subunit protein, normalised to β-actin loading control and expressed as optical density units (n = 5 for each group). Data are mean ± SEM. (D) Representative recording depicting loreclezole inhibition of GABAA receptor-mediated currents. Each bar represents application of 20 µm GABA. After stable peak amplitude response to GABA was achieved, 30 µm loreclezole was preapplied by local perfusion onto the cell for 30 s and loreclezole remained present through three subsequent GABA pulses applied in 30 s intervals. Following 60 s wash out of loreclezole, peak amplitude of GABA response was restored. A.U., arbitrary unit.