Abstract
Breakage of bilateral symmetry in amphibian embryos depends on the development of a ciliated epithelium at the gastrocoel roof during early neurulation. Motile cilia at the gastrocoel roof plate (GRP) give rise to leftward flow of extracellular fluids. Flow is required for asymmetric gene expression and organ morphogenesis. Wnt signaling has previously been involved in two steps, Wnt/ß-catenin mediated induction of Foxj1, a regulator of motile cilia, and Wnt/planar cell polarity (PCP) dependent cilia polarization to the posterior pole of cells. We have studied Wnt11b in the context of laterality determination, as this ligand was reported to activate canonical and non-canonical Wnt signaling. Wnt11b was found to be expressed in the so-called superficial mesoderm (SM), from which the GRP derives. Surprisingly, Foxj1 was only marginally affected in loss-of-function experiments, indicating that another ligand acts in this early step of laterality specification. Wnt11b was required, however, for polarization of GRP cilia and GRP morphogenesis, in line with the known function of Wnt/PCP in cilia-driven leftward flow. In addition Xnr1 and Coco expression in the lateral-most GRP cells, which sense flow and generate the first asymmetric signal, was attenuated in morphants, involving Wnt signaling in yet another process related to symmetry breakage in Xenopus.
Introduction
In vertebrates many inner organs of the chest and abdomen, such as heart and stomach, are asymmetrically localized along the left-right (LR) body axis [1]. Initiation of LR asymmetry in fish, amphibians and mammals is achieved by a cilia-driven leftward flow of extracellular fluids during neurulation [2]–[4]. Ciliated epithelia exist only very transiently and are represented by the amphibian GRP [5], Kupffer’s vesicle in fish [6] and posterior notochord (“node”) in mammals [7], [8]. The lateral margins of these epithelia are characterized by cells which co-express the growth factor nodal (Xnr1 in Xenopus) as well as a nodal inhibitor (Coco in Xenopus) [9]–[12]. As a result of asymmetric flow, the expression of the nodal inhibitor on the left side is down-regulated, which is thought to release nodal repression and initiate the nodal-cascade exclusively in the left lateral plate mesoderm [9].
Wnt signaling is a highly complex and multiple branched signaling pathway, which plays a plethora of important roles during animal development, tissue homeostasis and in human disease [13]–[15]. During LR axis development the canonical Wnt/β-catenin pathway initiates the expression of the transcription factor Foxj1, a master regulator of motile cilia, in the SM [16]–[18]. The SM represents a part of the epithelial outer layer of the gastrula embryo. It neighbors the organizer caudally and involutes during gastrulation to give rise to the GRP [19]. Foxj1 expression in Kupffer’s vesicle of zebrafish embryos is also regulated by Wnt/β-catenin [20], indicating conserved Wnt-dependency of Foxj1 expression during LR axis development. The non-canonical Wnt/PCP pathway was shown to be necessary for cilia polarization to the posterior pole of GRP cells [21], as a prerequisite for the generation of a directed laminar flow from right to left [16], [22]. This role of Wnt/PCP for LR axis specification was also described in mouse [23], [24], arguing for evolutionary conservation of this Wnt-dependent step in LR development as well.
In zebrafish the ligands Wnt3a, Wnt8 and Wnt11 were shown to be required for LR development [20], [25], [26]. In Xenopus, Wnt8a is not expressed in the SM or GRP [27], [28]. Wnt3a expression only starts at stages when Foxj1 is already expressed in the SM [29], [30]. Wnt11b, in contrast, is present in the oocyte and zygotic expression persists in dorsal regions before and after the onset of gastrulation [29]–[31]. Wnt11b can activate both canonical and non-canonical signaling branches during Xenopus development [32]–[34]. Maternally deposited Wnt11b mRNA is enriched on the dorsal side during cleavage stages and contributes to organizer formation by activation of Wnt/β-catenin signaling [31], [35]. During gastrulation and later development, Wnt11b and Wnt11r regulate convergent extension [36]–[38], neural crest cell induction and migration [39]–[41] as well as heart [42] and pronephric development [43] by activation of non-canonical Wnt signaling branches, i.e. Wnt/PCP and Wnt/calcium signaling [44], [45]. Wnt11b was therefore analyzed for a potential role in Wnt/β-catenin dependent Foxj1 expression and Wnt/PCP dependent cilia polarization during Xenopus LR development.
Results
Wnt11b is Expressed in the Superficial Mesoderm
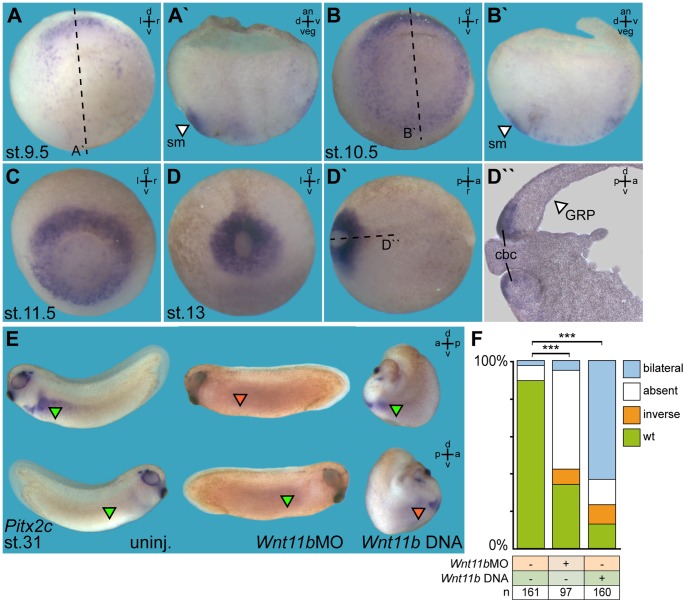
As a first step to elucidate the role of Wnt11b during LR axis development we analyzed mRNA expression patterns at LR relevant sites (Figure 1A–D). With the onset of gastrulation (stage 9.5) zygotic Wnt11b expression started in the dorsal region of the prospective mesoderm (Figure 1A). Manual bisection of embryos revealed expression in the SM, but not in deeper layers of the organizer (Figure 1A′), reminiscent of Foxj1 expression (Figure S1 A, A′). At stage 10.5 the domain expanded laterally (Figure 1B), eventually forming a ring of expression around the blastopore by stage 11.5 (Figure 1C). In dorsal regions the expression remained restricted to the SM, while mRNA in more lateral and ventral regions was detected in deep mesodermal cells as well (Figure 1B′). By stage 13, when the blastopore is closing, Wnt11b was expressed within the circumblastoporal collar (Figure 1D–D″), i.e. a ring of cells which involute into the gastrocoel. These expression patterns support a possible role of Wnt11b during GRP formation and LR development.
Figure 1. Wnt11b expression in the superficial mesoderm is required for asymmetric Pitx2c expression in the lateral plate mesoderm.
(A–D) Wnt11b mRNA expression as determined by whole-mount in situ hybridization of staged embryos at stage (st.) 9.5 (A), st. 10.5 (B), st. 11.5 (C) and st. 13 (D). Specimens are shown in vegetal (A–D) or dorsal (D′) view. Please note that Wnt11b mRNA was present in the superficial mesoderm (sm; white arrowheads) on the dorsal side, as shown in bisected embryos in (A′) and (B′), while expression on the ventral side extended into deeper tissue (B′). Note also expression in the circumblastular collar (cbc) of involuting cells, as demonstrated in a histological section (D″). (E, F) Altered Pitx2c expression in Wnt11b-manipulated embryos. (E) Representative specimens. (F) Quantification of results. Green arrowheads, wild-type expression; red arrowheads, ectopic or absent expression. Dashed lines in (A, B, D′) indicate planes of section. *** Very highly significant (p<0.001). a = anterior, an = animal, d = dorsal, l = left, n = number, p = posterior, r = right, uninj. = uninjected, v = ventral, veg = vegetal.
Wnt11b-manipulated Embryos show Loss of Asymmetric Pitx2c Expression
Wnt11b function in LR development was addressed by morpholino oligonucleotide (MO) mediated knockdown [50], [51] of Wnt11b translation and in gain-of-function experiments using a full length Wnt11b DNA expression construct. Short of a Xenopus Wnt11b-specific antibody, knockdown efficiencies could not be addressed directly. SM and GRP were targeted by injecting 4-cell embryos into the dorsal marginal zone [52]. Specimens were cultured until they reached stage 31 and analyzed for Pitx2c gene expression by whole mount in situ hybridization [2], [53]–[55]. Remarkably, both gain and loss of Wnt11b function resulted predominantly in loss of asymmetric Pitx2c expression in the left LPM (Figure 1E, F). In Wnt11b morphants Pitx2c expression was mostly absent, while bilateral expression of Pitx2c represented the most frequently encountered phenotype following Wnt11b DNA injection (Figure 1E, F). Ectopic expression of Wnt11b in addition resulted in severely shortened anterior-posterior axes (Figure 1E), indicative of convergent extension defects [56].
Next we asked whether changes in Foxj1 expression might correlate with absence of Pitx2c expression in morphants. Unexpectedly, differences from the wildtype pattern were recorded only in a minority of cases (Figure S1B, C). As both organizer function and notochord formation are required for normal LR development, we analyzed Xnr3 and Not mRNA expression in Wnt11b morphants [18], [57], [58]. Both were expressed in wildtype fashion (Figure S1D, E), demonstrating that organizer and notochord were not affected.
In order to test whether ligand-mediated Wnt signaling was indeed required for Foxj1 induction in the SM, we used a previously characterized antisense MO to interfere with translation of the canonical Wnt receptor Frizzled 8 (Fz8), which was shown to be active on the dorsal side of the Xenopus gastrula [59], [60]. As shown in Figure S1 (F, G), Foxj1 expression in the SM was severely affected in Fz8 morphants, and down-regulation was rescued by co-injection of a β-catenin DNA expression construct. These experiments confirm our previous results that ligand-mediated canonical Wnt signaling is required for Foxj1 expression in the SM [16]. Wnt11b thus is required in a Foxj1 independent manner in Wnt-dependent LR axis development.
Manipulation of Wnt11b Perturbs Leftward Flow at the GRP
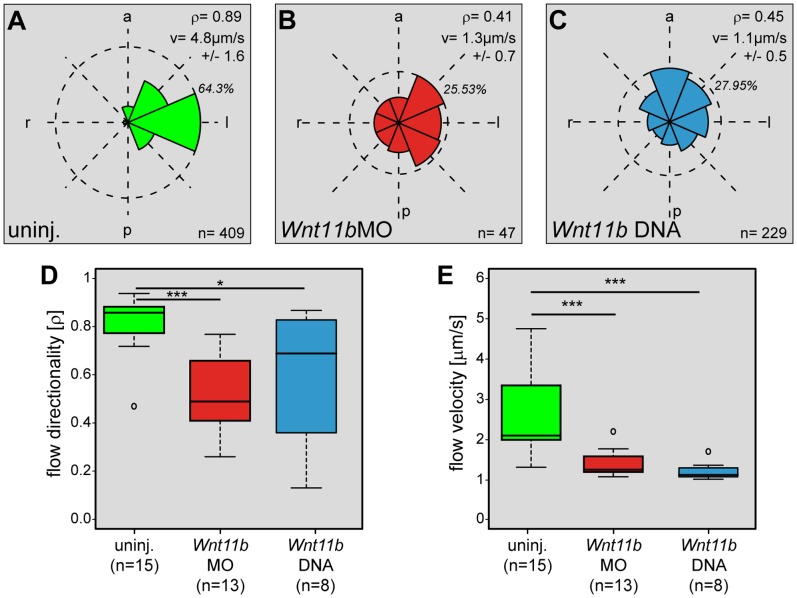
In an attempt to systematically dissect the LR pathway in Wnt11b manipulated embryos, we turned to leftward flow as the next step downstream from SM specification and Foxj1 induction. Directionality and velocity of flow were analyzed in dorsal explants of Wnt11bMO and Wnt11b DNA injected specimens (Figure 2 and Movie S1). Robust leftward flow was detected in uninjected control explants (Figure 2A), while flow directionality was compromised in Wnt11b morphants (Figure 2B) as well as following Wnt11b DNA injection (Figure 2C). To evaluate flow in groups of manipulated specimens we used the dimensionless number rho (ρ), which provides a qualitative measure (Figure 2D). Rho was calculated from time-lapse movies and represents the mean resultant directionality of particle trails (Rayleigh’s test of uniformity) [5]. Rho values range from 1, when all trajectories point in the same direction, to 0, when particles move randomly. Explants from uninjected control embryos showed a mean ρ-value of 0.82±0.12, while flow in Wnt11bMO and Wnt11b DNA injected specimens reached ρ-value of 0.51±0.17 and 0.6±0.28, respectively. While these data clearly demonstrate the impact of Wnt11b knockdown on flow directionality, the residual flow has not lost directionality altogether. Flow velocities were calculated from the same set of time-lapse movies (Figure 2E). Velocities in Wnt11b morphants and following Wnt11b DNA injection were found at 1.37µm/s±0.32 and 1.19µm/s±0.23, respectively, compared to uninjected controls which displayed a mean velocity of 2.54µm/s±0.94 (Figure 2E). Velocity thus was affected in a more pronounced manner than flow directionalty. These data pinpoint flow as a decisive step for Wnt11b function during LR development.
Figure 2. Wnt11b is required for leftward flow at the GRP.
Flow analysis in wildtype and manipulated specimens. (A–C) Frequency distribution of trajectory angles in representative explants of uninjected control embryos (uninj., A), Wnt11bMO (B) and Wnt11b DNA (C) injected specimens. Dashed circles indicate maximum frequency (in %), n represents the number of tracked particles above threshold. a = anterior, l = left, p = posterior, r = right, v = average velocity of particles, ρ = quality of flow. (D, E) Compiled results of all embryos analyzed for flow directionality (D) and velocity of fluorescent beads added to GRP explants at stage 17 (E). Note that both parameters were significantly reduced in Wnt11b morphants or Wnt11b DNA injected embryos. In (D, E), n represents number of analyzed explants. * Significant (p<0.05), ***Very highly significant (p<0.001), n = number of analyzed explants.
Wnt11b Regulates Wnt/PCP Dependent Cilia Polarization and Morphogenesis of the GRP
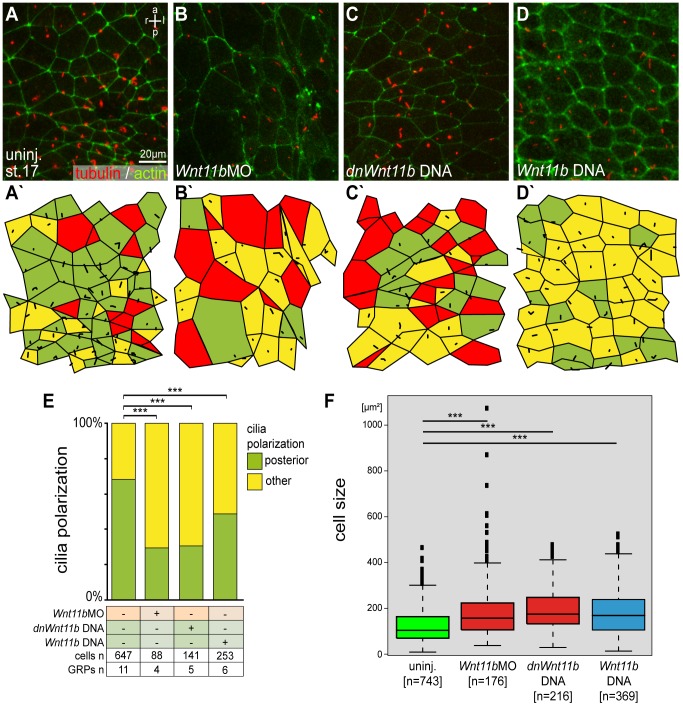
Next we analyzed cilia polarization, a process known to depend on Wnt/PCP [21]–[24]. GRP explants from Wnt11b manipulated embryos were stained for cilia and cell borders using an antibody against acetylated tubulin and phalloidin (Figure 3A–D). In uninjected control GRPs most cells were ciliated (79%) and cilia were localized to the posterior pole (Figure 3A′, E). Analysis of Wnt11b morphants and embryos injected with DNA encoding either a dominant negative Wnt11b construct (dnWnt11b; [37]), which is specific for Wnt5/11-type ligands without affecting the canonical pathway, or wildtype Wnt11b revealed disturbed cilia polarization (Figure 3B′–D′ and E). Remarkably, clear differences were seen between loss-of-function scenarios and ectopic expression of Wnt11b. The ciliation rate was reduced to 50% and 66% in Wnt11b morphants and following injection of dnWnt11b DNA, respectively. Overexpression of Wnt11b did not alter the wildtype ciliation rate of about 80%, but cilia were predominantly unpolarized, i.e. arose in a central position (Figure 3D′, E). In addition we observed that the apical surface of GRP cells was enlarged upon Wnt11b manipulation (Figure 3F). Average surface areas measured 193.71µm2±143.00, 195.79µm2±92.33 and 178.64µm2±95.73 in Wnt11bMO, dnWnt11b DNA and Wnt11b DNA injected specimens, respectively, compared to 123.88µm2±74.28 in control specimens, indicating an effect on GRP cell morphogenesis (Figure 3F). Taken together, balanced levels of Wnt11b seem to be required for Wnt/PCP dependent cilia polarization and GRP morphogenesis.
Figure 3. Wnt11b is required for cilia polarization and GRP morphogenesis.
Embryos were injected at the 4-cell stage into the prospective dorsal marginal zone and dorsal explants were prepared at stage 17. Specimens were processed for immunohistochemistry (IHC) to assess cilia polarization, ciliation rate and cell surface area. (A–D) Presence and polarization of cilia, as shown by acetylated tubulin IHC to stain cilia (red) and phalloidin to stain actin (green) in order to outline cell boundaries. (A) Control uninjected (uninj.) specimen. (B) Wnt11b morphant. (C) Specimen injected with dominant-negative Wnt11b DNA (dnWnt11b). (D) Specimen injected with wild-type Wnt11b DNA. (A′–D′) Evaluation of ciliation and polarization. Green = posterior localization of cilia, yellow = other localization, red = cells without cilia. (E, F) Evaluation of results. (E) Cilia polarization. (F) Apical cell surface area. ***Very highly significant (p<0.001). a = anterior, l = left, p = posterior, r = right.
Loss of Wnt11b Disrupts Xnr1 and Coco Expression in Lateral Sensory GRP Cells
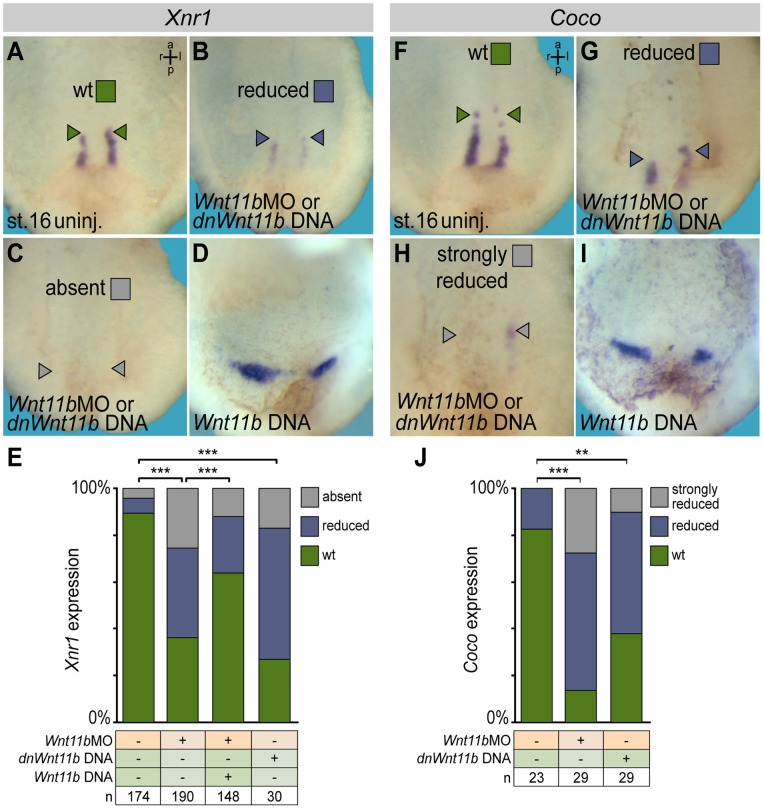
GRP analyses implemented Wnt11b in the LR cascade at the level of flow or events downstream. They do, however, not provide an explanation as to the opposing effects of Wnt11b manipulation on Pitx2c expression, namely absence in morphants and bilateral induction upon ectopic expression (cf. Figure 1E, F). Our previous analysis of ATP4a has shown that a turbulent and attenuated cilia-driven flow is sufficient to induce the nodal-cascade in a bilateral fashion [16], in line with the characterization of Wnt11b DNA injected specimens presented here (Figure 1E, F and 2C). In order to elucidate the opposing effect in morphants, we analyzed the lateral GRP cells which express both Xnr1 and its inhibitor Coco (Figure 4A, F), and which are required for LPM Xnr1 induction [9]. Wnt11b morphants and specimens injected with dnWnt11b showed significantly reduced expression levels of both genes (Figure 4B, C, G, H). Ectopic expression of Wnt11b, in contrast, showed comparable signal strength to wildtype specimens (Figure 4D, I), although domains were not aligned in parallel due to more pronounced convergent-extension phenotypes encountered in these experiments (cf. Figure 1E). Specificity of treatments was confirmed by co-injection of Wnt11b DNA in Wnt11b morphants, which partially restored Xnr1 expression (Figure 4E). These differential effects on Xnr1/Coco provide an explanation for LPM Pitx2c induction in the various experiments. Taken together, our data involve Wnt11b in the setup of the GRP and leftward flow.
Figure 4. Altered Xnr1 and Coco expression in Wnt11b manipulated embryos.
Embryos were injected at the 4-cell stage into the DMZ and analyzed for Xnr1 (A–E) or Coco expression (F–J) by whole mount in situ hybridization. Wildtype expression patterns (A, F) were reduced (B, G) or absent/strongly reduced (C, H) in Wnt11b morphants and in embryos injected with a dnWnt11b DNA construct, while signal intensities were unaltered upon ectopic wildtype Wnt11b expression from a DNA construct (D, I). (E, J) Quantification of results. Note that co-injection of wild-type Wnt11b DNA was sufficient to partially rescue Xnr1 expression at the GRP of Wnt11b morphants. ***Very highly significant (p<0.001). a = anterior, l = left, p = posterior, r = right.
Discussion
A role of Wnt signaling in LR axis development has been previously demonstrated in vertebrate model organisms including Xenopus [16], [20]–[26], [49], [61]–[70]. The sequential activity of two Wnt pathway branches is required for cilia-driven leftward flow: (1) canonical Wnt/β-cat signaling regulates Foxj1 expression during gastrulation in the Xenopus SM and in zebrafish Kupffer’s vesicle [16], [20]; (2) non-canonical Wnt/PCP signaling is required for the posterior alignment of motile cilia at the frog GRP and the posterior notochord in mouse [16], [21], [23], [24]. The present work confirmed the Fz8-mediated Wnt/β-catenin dependent activation of Foxj1 expression in the SM [16]. Wnt11b, however, contributes only marginally, if at all, to this process. Two additional canonical Wnt ligands are expressed during Xenopus gastrulation, Wnt3a and Wnt8a [27]–[30]. Wnt3a expression starts only after the onset of Foxj1 transcription in the SM. It is therefore tempting to speculate that Wnt8a represents the main canonical activator of Foxj1 expression during gastrulation. This notion may seem contra-intuitive at first glance, as Wnt8a is expressed in ventral and lateral portions of the prospective mesoderm but not in the SM itself [27]–[28]. We have, however, previously shown that ventral and lateral portions of the mesodermal ring are competent to express Foxj1 upon activation of canonical Wnt signaling [16]. Restriction of Foxj1 expression to the SM thus might be mediated by expression of the receptor Fz8.
Our study clearly demonstrates a role of Wnt11b in Wnt/PCP dependent cilia polarization at the GRP. Cilia alignment was altered by both gain and loss of non-canonical Wnt11b signaling, in agreement with findings in other systems in which non-canonical Wnt signaling was manipulated [16], [21], [23], [24]. Although the role of Wnt/PCP in Vangl2-dependent cilia polarization is well established [21]–[24], the initial global cue(s) for posterior orientation of cilia in vertebrate flow-generating epithelia has not been identified as yet. Wnt11b is expressed in the circumblastoporal collar, i.e. in cells en route to involute into the gastrocoel. Cells expressing Wnt11b mRNA therefore likely start secreting the protein following involution, i.e. when they are localized at the posterior margin of the GRP. Such a localization might establish a posterior to anterior gradient of Wnt11b protein, which, together with the co-expressed non-canonical ligand Wnt5a [32], [37], [38] might serve as instructive cue for cilia polarization at the GRP. Wnt gradients might mediate asymmetric phosphorylation of Vangl2, leading to anterior-posterior asymmetric localization of motile cilia, similar to the polarization mechanism proposed in the mouse limb bud [71].
Remarkably, we found another Wnt-dependent process during LR axis formation in Xenopus, namely Xnr1/Coco expression at the lateral-most aspects of the GRP. Previous reports have implicated canonical Wnt/β-cat signaling in the expression of the Xnr1/Coco homologs spaw/charon in zebrafish [25], [62] and in the homologous nodal expression domain in mouse [65]. Canonical Wnt signaling is important for organizer formation and function, which in turn is required for correct LR axis development [57]. The observed effects on nodal expression therefore might be indirect. The unaltered Xnr3 and Not expression patterns in our Wnt11b morphants and the effectiveness of a dnWnt11b DNA construct, which is only activated post MBT [72], however, argue for a specific impact of Wnt signaling on lateral cells of the GRP, unrelated to organizer function and notochord formation.
We have recently shown that ATP4a is required for Wnt/β-catenin and Wnt/PCP signaling during Xenopus LR development [16], similar to ATP6 [73]–[75]. It seems unlikely that Wnt11b acts on Xnr1/Coco expression via the Wnt/β-catenin or Wnt/PCP pathways, because morpholino-mediated loss of ATP4a function did not affect Xnr1 or Coco expression. Furthermore, pharmacological inhibition of ATP6 during frog, chick and zebrafish development did not lead to a loss, but randomized Xnr1, nodal or spaw expression, respectively [76].
Which signaling branch might Wnt11b act on in the context of LR development? We like to propose an involvement of Wnt/calcium signaling. Wnt11b is known to interact with the Wnt/calcium pathway during Xenopus development, especially during gastrulation [43], [45], [77]. Manipulation of calcium signaling during gastrulation alters LR development in zebrafish, Xenopus and mouse [63], [78]–[81], in line with a possible role of Wnt/calcium signaling in the regulation of Xnr1 and Coco expression. Further experiments are required in the various model organisms to resolve the precise mechanism of Wnt-dependent expression of nodal and its respective inhibitor in the lateral flow-sensing cells of the ciliated organs of laterality.
Materials and Methods
Ethics Statement
All animals were treated according to the German regulations and laws for care and handling of research animals, and experimental manipulations according to §6, article 1, sentence 2, nr. 4 of the animal protection act were approved by the Regional Government Stuttgart, Germany (Vorhaben A 365/10 ZO “Molekulare Embryologie”).
Statistical Evaluation of Results
Statistical evaluation of experiments represented by bar graphs was performed using chi-square tests (http://www.physics.csbsju.edu/stats/contingency.html). In Figure 1F, the number of manipulated embryos (Wnt11bMO or Wnt11b DNA) displaying wt, inverse, bilateral or absent Pitx2c expression was compared to the numbers of embryos displaying these expression patterns in uninjected control specimens. In Figure S1C, F, the number of morphant embryos with wt, reduced and absent Foxj1 expression was compared to the number of embryos with wt, reduced or absent expression in uninjected controls and embryos co-injected with β-catenin DNA. In Figure 4E, J, the number of Wnt11bMO or dnWnt11b DNA injected embryos with wt, reduced or absent/strongly reduced expression of Xnr1 or Coco, respectively, was compared to the number of embryos with these characteristics in uninjected control specimens and Wnt11b morphants co-injected with wt Wnt11b DNA. Statistics of experiments represented by box plots were calculated by Wilcoxon sum of ranks (Mann-Whitney) tests (http://www.fon.hum.uva.nl/Service/Statistics/Wilcoxon_Test.html).
Manipulation of Embryos
Embryos were injected at the two- to four-cell stage using a Harvard Apparatus setup in 1× modified Barth’s solution (MBSH) with 4% Ficoll (BioChemica) and transferred to 0.1× MBSH 15 min after injection. Drop size was calibrated to about 7–8 nl per injection. Rhodamine-B or Cascade blue dextran (0.5–1.0 mg/ml; Molecular Probes) were coinjected and used as lineage tracer. Wnt11bMO (5′-TAACCCAGTGACGGGTCGGAGCCAT-3′) was used at 1–2 pmol per embryo, Fz8MO [46] at 2 pmol per specimen. DNAs were purified using the PureYield Plasmid Midiprep kit (Promega) and diluted to a concentration of 1 ng/µl (dnWnt11b CS2+ [38]), 0.5–1 ng/µl (Wnt11b CS2+ [38]) and 1ng/µl (β-cat:GFP [47]).
Whole-Mount In Situ Hybridization
Embryos were fixed in MEMFA for 1–2 hrs and processed following standard protocols. Digoxigenin-labeled (Roche) RNA probes were prepared from linearized plasmids using SP6, T3, or T7 RNA polymerase (Promega). In situ hybridization was conducted as previously described [16]. For histological analyses, embryos were embedded in gelatin-albumin and sectioned on a vibratome (30 µm).
Flow Analysis
Embryos were coinjected with lineage tracer to control for correct targeting of the GRP. Data processing was as previously described [5], [16], [48], [49]. The whiskers of the box plots extend to maximal 1.5×IQR, and outliers are displayed as circles.
Immunohistochemistry and GRP Analysis
Immunohistochemistry was performed as described [48] using Anti-Tubulin Acetylated (mouse, 1∶700; Sigma) and anti-mouse Cy3 (sheep, 1∶250; Sigma). Cell boundaries were visualized by Alexa 488-conjugated phalloidin (Invitrogen), which stained the actin cytoskeleton. Imaging was performed on a Zeiss LSM700. To determine GRP cell parameters, an area of 320×320 µm at the center of the GRP was selected for manual analysis of cilia number/polarization and GRP cell size using ImageJ [16], [49]. The whiskers of the box plots extend to maximal 1.5× IQR, outliers are displayed as circles.
Supporting Information
Foxj1 expression requires Wnt signaling through Fz8, but is largely independent of Wnt11b. (A, A′) Foxj1 expression in the superficial mesoderm at stage (st.) 10.5. in whole mount (A) and bisected specimens (A′). (B, C) Marginal effects on Foxj1 mRNA expression levels and localization in Wnt11b morphants (quantification in C). (D, E) Wildtype expression of Xnr3 (D) and Not (E) in Wnt11b morphant embryos. (F, G) Foxj1 expression requires Fz8. (F) Summary of results. (G) Altered Foxj1 expression in Fz8 morphants is partially rescued by co-injection of β-catenin (β-cat). Green arrowhead, wild-type expression; red arrowhead, reduced expression; gray arrowhead, absent expression. Dashed line in (A) indicates plane of bisection. ** Highly significant (p<0.01), *** Very highly significant (p<0.001). a = anterior, an = animal, d = dorsal, l = left, n = number, p = posterior, r = right, v = ventral, veg = vegetal.
(TIF)
Flow defects in GRP explants from Wnt11b manipulated embryos. Movie shows time-lapse sequences of dorsal explants to which fluorescent beads were added (cf. Figure 2A-C). Specimens were mounted dorsal side down and viewed from the ventral side, anterior to the top. Movie represents a total length of 500 frames taken at a rate of 2 frames/sec and runs at 40×real time. Opening frame displays bright field images and indicates orientation of GRP (dashed lines). Videos were processed to yield gradient time trails (GTTs), i.e. color-coded tracks of beads which revealed direction of transport and velocity of particles (from green to red; 25 s). Note that robust leftward flow (uninjected controls) was impaired in Wnt11b morphant and upon injection of wild-type Wnt11b DNA.
(MOV)
Acknowledgments
Valuable discussions with Jan Christian and Richard Harland are gratefully acknowledged. Tina Beyer, Thomas Thumberger and Bärbel Ulmer offered their continuous advice throughout the progress of the project. Anna Schäfer helped with some of the experiments. C. Kintner, R. Moon, M. Tada and W. Wu provided reagents.
Funding Statement
This work was supported by DFG Grant BL285/9-1 to MB. PW was recipient of a PhD fellowship from the Landesgraduiertenförderung Baden-Württemberg. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Basu B, Brueckner M (2008) Cilia multifunctional organelles at the center of vertebrate left-right asymmetry. 1st ed. Elesvier Inc. doi:10.1016/S0070-2153(08)00806-5. [DOI] [PubMed]
- 2.Schweickert A, Walentek P, Thumberger T, Danilchik M (2011) Linking early determinants and cilia-driven leftward flow in left-right axis specification of Xenopus laevis: A theoretical approach. Differentiation; research in biological diversity: 1–11. doi:10.1016/j.diff.2011.11.005. [DOI] [PubMed]
- 3. Blum M, Weber T, Beyer T, Vick P (2009) Evolution of leftward flow. Seminars in cell & developmental biology 20: 464–471 doi:10.1016/j.semcdb.2008.11.005 [DOI] [PubMed] [Google Scholar]
- 4. Hirokawa N, Tanaka Y, Okada Y, Takeda S (2006) Nodal flow and the generation of left-right asymmetry. Cell 125: 33–45 doi:10.1016/j.cell.2006.03.002 [DOI] [PubMed] [Google Scholar]
- 5. Schweickert A, Weber T, Beyer T, Vick P, Bogusch S, et al. (2007) Cilia-driven leftward flow determines laterality in Xenopus. Current biology?: CB 17: 60–66 doi:10.1016/j.cub.2006.10.067 [DOI] [PubMed] [Google Scholar]
- 6. Essner JJ, Amack JD, Nyholm MK, Harris EB, Yost HJ (2005) Kupffer’s vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development (Cambridge, England) 132: 1247–1260 doi:10.1242/dev.01663 [DOI] [PubMed] [Google Scholar]
- 7. Blum M, Andre P, Muders K, Schweickert A, Fischer A, et al. (2007) Ciliation and gene expression distinguish between node and posterior notochord in the mammalian embryo. Differentiation; research in biological diversity 75: 133–146 doi:10.1111/j.1432-0436.2006.00124.x [DOI] [PubMed] [Google Scholar]
- 8. Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada a, et al. (1998) Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95: 829–837. [DOI] [PubMed] [Google Scholar]
- 9. Schweickert A, Vick P, Getwan M, Weber T, Schneider I, et al. (2010) The Nodal Inhibitor Coco Is a Critical Target of Leftward Flow in Xenopus. Current Biology 20: 738–743. [DOI] [PubMed] [Google Scholar]
- 10. Vonica A, Brivanlou AH (2007) The left-right axis is regulated by the interplay of Coco, Xnr1 and derrière in Xenopus embryos. Developmental biology 303: 281–294 doi:10.1016/j.ydbio.2006.09.039 [DOI] [PubMed] [Google Scholar]
- 11. Hojo M, Takashima S, Kobayashi D, Sumeragi A, Shimada A, et al. (2007) Right-elevated expression of charon is regulated by fluid flow in medaka Kupffer’s vesicle. Development, growth & differentiation 49: 395–405 doi:10.1111/j.1440-169x.2007.00937.x [DOI] [PubMed] [Google Scholar]
- 12. Marques S, Borges AC, Silva AC, Freitas S, Cordenonsi M, et al. (2004) The activity of the Nodal antagonist Cerl-2 in the mouse node is required for correct L/R body axis. Genes & development 18: 2342–2347 doi:10.1101/gad.306504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. MacDonald BT, Tamai K, He X (2009) Wnt/beta-catenin signaling: components, mechanisms, and diseases. Developmental cell 17: 9–26 doi:10.1016/j.devcel.2009.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sugimura R, Li L (2010) Noncanonical Wnt signaling in vertebrate development, stem cells, and diseases. Birth defects research Part C, Embryo today?: reviews 90: 243–256 doi:10.1002/bdrc.20195 [DOI] [PubMed] [Google Scholar]
- 15. Yang Y (2012) Wnt signaling in development and disease. Cell & bioscience 2: 14 doi:10.1186/2045-3701-2-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walentek P, Beyer T, Thumberger T, Schweickert A, Blum M (2012) ATP4a Is Required for Wnt-Dependent Foxj1 Expression and Leftward Flow in Xenopus Left-Right Development. CellReports. doi:10.1016/j.celrep.2012.03.005. [DOI] [PubMed]
- 17. Stubbs JL, Oishi I, Izpisúa Belmonte JC, Kintner C (2008) The forkhead protein Foxj1 specifies node-like cilia in Xenopus and zebrafish embryos. Nature Genetics 40: 1–7 doi:10.1038/ng.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alten L, Schuster-Gossler K, Beckers A, Groos S, Ulmer B, et al. (2012) Differential regulation of node formation, nodal ciliogenesis and cilia positioning by Noto and Foxj1. Development (Cambridge, England) 1284: 1276–1284 doi:10.1242/dev.072728 [DOI] [PubMed] [Google Scholar]
- 19. Shook DR, Majer C, Keller R (2004) Pattern and morphogenesis of presumptive superficial mesoderm in two closely related species, Xenopus laevis and Xenopus tropicalis. Developmental biology 270: 163–185 doi:10.1016/j.ydbio.2004.02.021 [DOI] [PubMed] [Google Scholar]
- 20. Caron A, Xu X, Lin X (2012) Wnt/β-catenin signaling directly regulates Foxj1 expression and ciliogenesis in zebrafish Kupffer’s vesicle. Development (Cambridge, England) 524: 514–524 doi:10.1242/dev.071746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Antic D, Stubbs JL, Suyama K, Kintner C, Scott MP, et al. (2010) Planar cell polarity enables posterior localization of nodal cilia and left-right axis determination during mouse and Xenopus embryogenesis. PloS one 5: e8999 doi:10.1371/journal.pone.0008999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hashimoto M, Hamada H (2010) Translation of anterior-posterior polarity into left-right polarity in the mouse embryo. Current opinion in genetics & development 20: 433–437 doi:10.1016/j.gde.2010.04.002 [DOI] [PubMed] [Google Scholar]
- 23. Song H, Hu J, Chen W, Elliott G, Andre P, et al. (2010) Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature 466: 378–382 doi:10.1038/nature09129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Borovina A, Superina S, Voskas D, Ciruna B (2010) Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nature cell biology 12: 407–412 doi:10.1038/ncb2042 [DOI] [PubMed] [Google Scholar]
- 25. Lin X, Xu X (2009) Distinct functions of Wnt/beta-catenin signaling in KV development and cardiac asymmetry. Development (Cambridge, England) 136: 207–217 doi:10.1242/dev.029561 [DOI] [PubMed] [Google Scholar]
- 26. Oteiza P, Köppen M, Krieg M, Pulgar E, Farias C, et al. (2010) Planar cell polarity signalling regulates cell adhesion properties in progenitors of the zebrafish laterality organ. Development (Cambridge, England) 137: 3459–3468 doi:10.1242/dev.049981 [DOI] [PubMed] [Google Scholar]
- 27. Christian JL, McMahon Ja, McMahon aP, Moon RT (1991) Xwnt-8, a Xenopus Wnt-1/int-1-related gene responsive to mesoderm-inducing growth factors, may play a role in ventral mesodermal patterning during embryogenesis. Development (Cambridge, England) 111: 1045–1055. [DOI] [PubMed] [Google Scholar]
- 28. Sykes TG, Rodaway aR, Walmsley ME, Patient RK (1998) Suppression of GATA factor activity causes axis duplication in Xenopus. Development (Cambridge, England) 125: 4595–4605. [DOI] [PubMed] [Google Scholar]
- 29. Yanai I, Peshkin L, Jorgensen P, Kirschner MW (2011) Mapping gene expression in two Xenopus species: evolutionary constraints and developmental flexibility. Developmental cell 20: 483–496 doi:10.1016/j.devcel.2011.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bowes JB, Snyder Ka, Segerdell E, Jarabek CJ, Azam K, et al. (2010) Xenbase: gene expression and improved integration. Nucleic acids research 38: D607–12 doi:10.1093/nar/gkp953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, et al. (2005) Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell 120: 857–871 doi:10.1016/j.cell.2005.01.013 [DOI] [PubMed] [Google Scholar]
- 32. Cha S-W, Tadjuidje E, Tao Q, Wylie C, Heasman J (2008) Wnt5a and Wnt11 interact in a maternal Dkk1-regulated fashion to activate both canonical and non-canonical signaling in Xenopus axis formation. Development (Cambridge, England) 135: 3719–3729 doi:10.1242/dev.029025 [DOI] [PubMed] [Google Scholar]
- 33. Li Y, Rankin Sa, Sinner D, Kenny AP, Krieg Pa, et al. (2008) Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes & development 22: 3050–3063 doi:10.1101/gad.1687308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cha S-W, Tadjuidje E, White J, Wells J, Mayhew C, et al. (2009) Wnt11/5a complex formation caused by tyrosine sulfation increases canonical signaling activity. Current biology?: CB 19: 1573–1580 doi:10.1016/j.cub.2009.07.062 [DOI] [PubMed] [Google Scholar]
- 35. Kofron M, Birsoy B, Houston D, Tao Q, Wylie C, et al. (2007) Wnt11/beta-catenin signaling in both oocytes and early embryos acts through LRP6-mediated regulation of axin. Development (Cambridge, England) 134: 503–513 doi:10.1242/dev.02739 [DOI] [PubMed] [Google Scholar]
- 36. Tahinci E, Thorne Ca, Franklin JL, Salic A, Christian KM, et al. (2007) Lrp6 is required for convergent extension during Xenopus gastrulation. Development (Cambridge, England) 134: 4095–4106 doi:10.1242/dev.010272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smith JC, Conlon FL, Saka Y, Tada M (2000) Xwnt11 and the regulation of gastrulation in Xenopus. Philosophical transactions of the Royal Society of London Series B, Biological sciences 355: 923–930 doi:10.1098/rstb.2000.0627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tada M, Smith JC (2000) Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development (Cambridge, England) 127: 2227–2238. [DOI] [PubMed] [Google Scholar]
- 39. Matthews HK, Broders-Bondon F, Thiery JP, Mayor R (2008) Wnt11r is required for cranial neural crest migration. Developmental dynamics?: an official publication of the American Association of Anatomists 237: 3404–3409 doi:10.1002/dvdy.21758 [DOI] [PubMed] [Google Scholar]
- 40.Ulmer B, Hagenlocher C, Schmalholz S, Kurz S, Schweickert A, et al.. (2013) Calponin 2 Acts As an Effector of Noncanonical Wnt-Mediated Cell Polarization during Neural Crest Cell Migration. Cell Reports: 615–621. doi:10.1016/j.celrep.2013.02.015. [DOI] [PubMed]
- 41. Garriock RJ, Krieg Pa (2007) Wnt11-R signaling regulates a calcium sensitive EMT event essential for dorsal fin development of Xenopus. Developmental biology 304: 127–140 doi:10.1016/j.ydbio.2006.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Garriock RJ, D’Agostino SL, Pilcher KC, Krieg Pa (2005) Wnt11-R, a protein closely related to mammalian Wnt11, is required for heart morphogenesis in Xenopus. Developmental biology 279: 179–192 doi:10.1016/j.ydbio.2004.12.013 [DOI] [PubMed] [Google Scholar]
- 43. Tételin S, Jones Ea (2010) Xenopus Wnt11b is identified as a potential pronephric inducer. Developmental dynamics?: an official publication of the American Association of Anatomists 239: 148–159 doi:10.1002/dvdy.22012 [DOI] [PubMed] [Google Scholar]
- 44. Flaherty MP, Dawn B (2008) Noncanonical Wnt11 signaling and cardiomyogenic differentiation. Trends in cardiovascular medicine 18: 260–268 doi:10.1016/j.tcm.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Uysal-Onganer P, Kypta RM (2012) Wnt11 in 2011 - the regulation and function of a non-canonical Wnt. Acta physiologica (Oxford, England) 204: 52–64 doi:10.1111/j.1748-1716.2011.02297.x [DOI] [PubMed] [Google Scholar]
- 46. Satow R, Chan T, Asashima M (2004) The role of Xenopus frizzled-8 in pronephric development. Biochemical and biophysical research communications 321: 487–494 doi:10.1016/j.bbrc.2004.06.166 [DOI] [PubMed] [Google Scholar]
- 47. Miller JR, Moon RT (1997) Analysis of the signaling activities of localization mutants of beta-catenin during axis specification in Xenopus. The Journal of cell biology 139: 229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vick P, Schweickert A, Weber T, Eberhardt M, Mencl S, et al. (2009) Flow on the right side of the gastrocoel roof plate is dispensable for symmetry breakage in the frog Xenopus laevis. Developmental biology 331: 281–291 doi:10.1016/j.ydbio.2009.05.547 [DOI] [PubMed] [Google Scholar]
- 49.Beyer T, Danilchik M, Thumberger T, Vick P, Tisler M, et al.. (2011) Serotonin Signaling Is Required for Wnt-Dependent GRP Specification and Leftward Flow in Xenopus. Current Biology: 1–7. doi:10.1016/j.cub.2011.11.027. [DOI] [PubMed]
- 50.Moulton JD, Yan Y-L (2008) Using Morpholinos to control gene expression. Current protocols in molecular biology/edited by Frederick M Ausubel. [et al] Chapter 26: Unit 26.8. doi:10.1002/0471142727.mb2608s83. [DOI] [PMC free article] [PubMed]
- 51. Eisen JS, Smith JC (2008) Controlling morpholino experiments: don’t stop making antisense. Development 135: 1735–1743 doi:10.1242/dev.001115 [DOI] [PubMed] [Google Scholar]
- 52. Blum M, Beyer T, Weber T, Vick P, Andre P, et al. (2009) Xenopus, an ideal model system to study vertebrate left-right asymmetry. Developmental dynamics?: an official publication of the American Association of Anatomists 238: 1215–1225 doi:10.1002/dvdy.21855 [DOI] [PubMed] [Google Scholar]
- 53. Shiratori H, Sakuma R, Watanabe M, Hashiguchi H, Mochida K, et al. (2001) Two-step regulation of left-right asymmetric expression of Pitx2: initiation by nodal signaling and maintenance by Nkx2. Molecular cell 7: 137–149. [DOI] [PubMed] [Google Scholar]
- 54. Ryan a K, Blumberg B, Rodriguez-Esteban C, Yonei-Tamura S, Tamura K, et al. (1998) Pitx2 determines left-right asymmetry of internal organs in vertebrates. Nature 394: 545–551 doi:10.1038/29004 [DOI] [PubMed] [Google Scholar]
- 55. Schweickert a, Steinbeisser H, Blum M (2001) Differential gene expression of Xenopus Pitx1, Pitx2b and Pitx2c during cement gland, stomodeum and pituitary development. Mechanisms of development 107: 191–194. [DOI] [PubMed] [Google Scholar]
- 56. Wallingford JB, Fraser SE, Harland RM (2002) Convergent extension: the molecular control of polarized cell movement during embryonic development. Developmental cell 2: 695–706. [DOI] [PubMed] [Google Scholar]
- 57. Danos MC, Yost HJ (1995) Linkage of cardiac left-right asymmetry and dorsal-anterior development in Xenopus. Development (Cambridge, England) 121: 1467–1474. [DOI] [PubMed] [Google Scholar]
- 58. Von Dassow G, Schmidt JE, Kimelman D (1993) Induction of the Xenopus organizer: expression and regulation of Xnot, a novel FGF and activin-regulated homeo box gene. Genes & Development 7: 355–366 doi:10.1101/gad.7.3.355 [DOI] [PubMed] [Google Scholar]
- 59. Itoh K, Jacob J, Sokol SY (1998) A role for Xenopus Frizzled 8 in dorsal development. Mechanisms of development 74: 145–157. [DOI] [PubMed] [Google Scholar]
- 60. Deardorff Ma, Tan C, Conrad LJ, Klein PS (1998) Frizzled-8 is expressed in the Spemann organizer and plays a role in early morphogenesis. Development (Cambridge, England) 125: 2687–2700. [DOI] [PubMed] [Google Scholar]
- 61. Walentek P, Beyer T, Thumberger T, Schweickert A, Blum M (2012) ATP4a Is Required for Wnt-Dependent Foxj1 Expression and Leftward Flow in Xenopus Left-Right Development. Cell Reports 1: 516–527 doi:10.1016/j.celrep.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 62. Zhang M, Zhang J, Lin S-C, Meng A (2012) β-Catenin 1 and β-catenin 2 play similar and distinct roles in left-right asymmetric development of zebrafish embryos. Development (Cambridge, England) 139: 2009–2019 doi:10.1242/dev.074435 [DOI] [PubMed] [Google Scholar]
- 63. Schneider I, Houston DW, Rebagliati MR, Slusarski DC (2008) Calcium fluxes in dorsal forerunner cells antagonize beta-catenin and alter left-right patterning. Development (Cambridge, England) 135: 75–84 doi:10.1242/dev.004713 [DOI] [PubMed] [Google Scholar]
- 64. Schneider I, Schneider PN, Derry SW, Lin S, Barton LJ, et al. (2010) Zebrafish Nkd1 promotes Dvl degradation and is required for left-right patterning. Developmental biology 348: 22–33 doi:10.1016/j.ydbio.2010.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nakaya M, Biris K, Tsukiyama T, Jaime S, Rawls JA, et al. (2005) Wnt3a links left-right determination with segmentation and anteroposterior axis elongation. Development (Cambridge, England) 132: 5425–5436 doi:10.1242/dev.02149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang Y, Levin M (2009) Left-right asymmetry in the chick embryo requires core planar cell polarity protein Vangl2. Genesis (New York, NY: 2000) 47: 719–728 doi:10.1002/dvg.20551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mahaffey JP, Grego-Bessa J, Liem KF, Anderson KV (2013) Cofilin and Vangl2 cooperate in the initiation of planar cell polarity in the mouse embryo. Development (Cambridge, England) 140: 1262–1271 doi:10.1242/dev.085316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bajoghli B, Aghaallaei N, Soroldoni D, Czerny T (2007) The roles of Groucho/Tle in left-right asymmetry and Kupffer’s vesicle organogenesis. Developmental biology 303: 347–361 doi:10.1016/j.ydbio.2006.11.020 [DOI] [PubMed] [Google Scholar]
- 69. Rodríguez-Esteban C, Capdevila J, Kawakami Y, Izpisúa Belmonte JC (2001) Wnt signaling and PKA control Nodal expression and left-right determination in the chick embryo. Development (Cambridge, England) 128: 3189–3195. [DOI] [PubMed] [Google Scholar]
- 70. Nascone N, Mercola M (1997) Organizer induction determines left-right asymmetry in Xenopus. Developmental biology 189: 68–78 doi:10.1006/dbio.1997.8635 [DOI] [PubMed] [Google Scholar]
- 71. Gao B, Song H, Bishop K, Elliot G, Garrett L, et al. (2011) Wnt Signaling Gradients Establish Planar Cell Polarity by Inducing Vangl2 Phosphorylation through Ror2. Developmental cell 20: 163–176 doi:10.1016/j.devcel.2011.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Newport J, Kirschner M (1982) A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell 30: 675–686. [DOI] [PubMed] [Google Scholar]
- 73. Buechling T, Bartscherer K, Ohkawara B, Chaudhary V, Spirohn K, et al. (2010) Wnt/Frizzled signaling requires dPRR, the Drosophila homolog of the prorenin receptor. Current Biology 20: 1263–1268 doi:10.1016/j.cub.2010.05.028 [DOI] [PubMed] [Google Scholar]
- 74.Cruciat C, Mediated VH, Ohkawara B, Acebron SP, Karaulanov E (2011) Requirement of Prorenin Receptor and Vacuolar H+-ATPase − Mediated Acidification for Wnt Signaling. Science 459. doi:10.1126/science.1179802. [DOI] [PubMed]
- 75. Niehrs C, Boutros M (2010) Trafficking, acidification, and growth factor signaling. Science signaling 3: pe26 doi:10.1126/scisignal.3134pe26 [DOI] [PubMed] [Google Scholar]
- 76. Adams DS, Robinson KR, Fukumoto T, Yuan S, Albertson RC, et al. (2006) Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development (Cambridge, England) 133: 1657–1671 doi:10.1242/dev.02341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kühl M, Sheldahl LC, Malbon CC, Moon RT (2000) Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. The Journal of biological chemistry 275: 12701–12711. [DOI] [PubMed] [Google Scholar]
- 78. Hatayama M, Mikoshiba K, Aruga J (2011) IP(3) signaling is required for cilia formation and left-right body axis determination in Xenopus embryos. Biochemical and biophysical research communications 410: 520–524 doi:10.1016/j.bbrc.2011.06.014 [DOI] [PubMed] [Google Scholar]
- 79. Kreiling J a, Balantac ZL, Crawford AR, Ren Y, Toure J, et al. (2008) Suppression of the endoplasmic reticulum calcium pump during zebrafish gastrulation affects left-right asymmetry of the heart and brain. Mechanisms of development 125: 396–410 doi:10.1016/j.mod.2008.02.004 [DOI] [PubMed] [Google Scholar]
- 80. Lai S-L, Yao W-L, Tsao K-C, Houben AJS, Albers HMHG, et al. (2012) Autotaxin/Lpar3 signaling regulates Kupffer’s vesicle formation and left-right asymmetry in zebrafish. Development (Cambridge, England) 139: 4439–4448 doi:10.1242/dev.081745 [DOI] [PubMed] [Google Scholar]
- 81. Takao D, Nemoto T, Abe T, Kiyonari H, Kajiura-Kobayashi H, et al. (2013) Asymmetric distribution of dynamic calcium signals in the node of mouse embryo during left-right axis formation. Developmental biology 376: 23–30 doi:10.1016/j.ydbio.2013.01.018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Foxj1 expression requires Wnt signaling through Fz8, but is largely independent of Wnt11b. (A, A′) Foxj1 expression in the superficial mesoderm at stage (st.) 10.5. in whole mount (A) and bisected specimens (A′). (B, C) Marginal effects on Foxj1 mRNA expression levels and localization in Wnt11b morphants (quantification in C). (D, E) Wildtype expression of Xnr3 (D) and Not (E) in Wnt11b morphant embryos. (F, G) Foxj1 expression requires Fz8. (F) Summary of results. (G) Altered Foxj1 expression in Fz8 morphants is partially rescued by co-injection of β-catenin (β-cat). Green arrowhead, wild-type expression; red arrowhead, reduced expression; gray arrowhead, absent expression. Dashed line in (A) indicates plane of bisection. ** Highly significant (p<0.01), *** Very highly significant (p<0.001). a = anterior, an = animal, d = dorsal, l = left, n = number, p = posterior, r = right, v = ventral, veg = vegetal.
(TIF)
Flow defects in GRP explants from Wnt11b manipulated embryos. Movie shows time-lapse sequences of dorsal explants to which fluorescent beads were added (cf. Figure 2A-C). Specimens were mounted dorsal side down and viewed from the ventral side, anterior to the top. Movie represents a total length of 500 frames taken at a rate of 2 frames/sec and runs at 40×real time. Opening frame displays bright field images and indicates orientation of GRP (dashed lines). Videos were processed to yield gradient time trails (GTTs), i.e. color-coded tracks of beads which revealed direction of transport and velocity of particles (from green to red; 25 s). Note that robust leftward flow (uninjected controls) was impaired in Wnt11b morphant and upon injection of wild-type Wnt11b DNA.
(MOV)