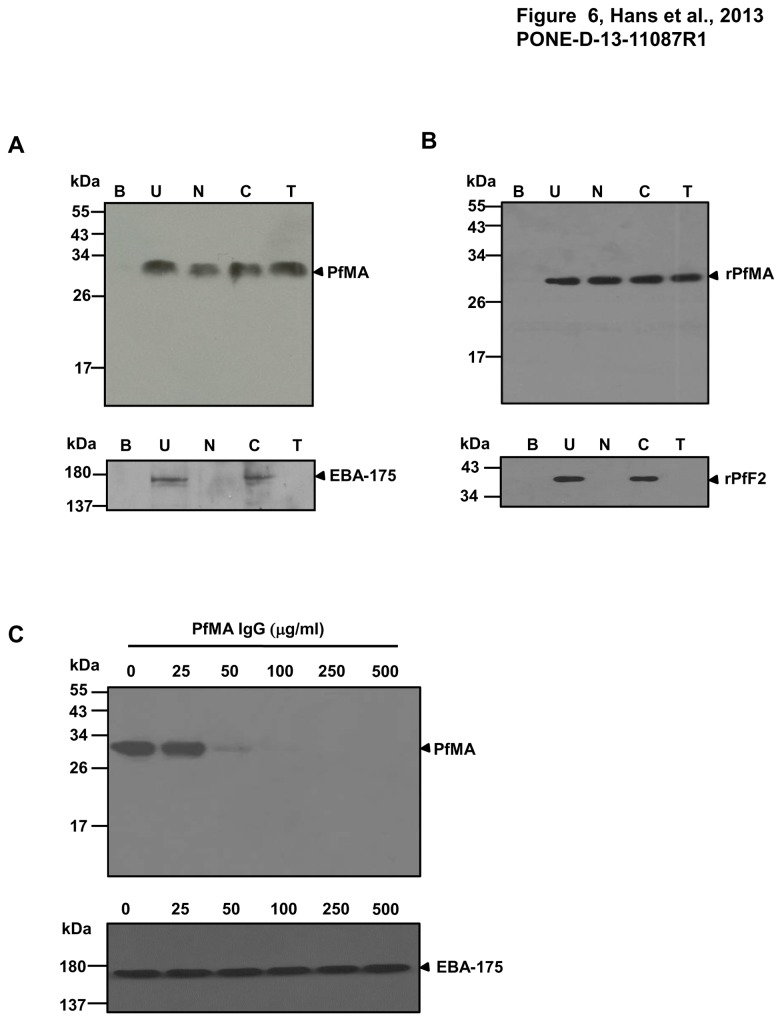
Figure 6. PfMA exhibits erythrocyte binding activity and anti-rPfMA antibodies blocked the erythrocyte binding of native PfMA.
(A) The erythrocyte binding activity of native PfMA was analyzed with a panel of untreated and different enzymatically treated erythrocytes. The ~32 kDa PfMA protein from parasite culture supernatants bound to untreated (U), neuraminidase (N), chymotrypsin (C) and trypsin (T) treated erythrocytes. Binding of native PfEBA-175 from the same parasite culture supernatants was analyzed as a positive control. (B) Recombinant rPfMA exhibited a similar binding specificity with the same set of enzymatically treated erythrocytes. Binding of rPfF2 was analyzed as a positive control. No PfMA protein was detected when the erythrocytes were incubated with an RPMI only control (B). (C) Anti-rPfMA antibodies specifically blocked erythrocyte binding of the native PfMA protein. 3D7 culture supernatant was incubated with normal erythrocytes in the presence of purified rabbit anti-PfMA IgG at different concentrations of 0–500 µg/µl. Anti-PfMA IgG had no effect on the binding of the native EBA-175 parasite protein.