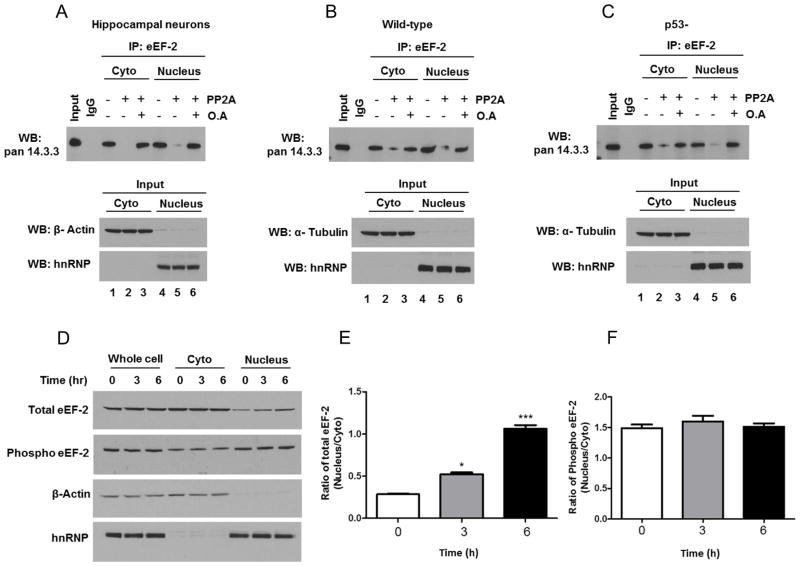
Figure 7.
Evidence for the differential involvement of 14-3-3 protein and CRM1 in the subcellular localization of total and phosphorylated eEF-2. A– C. Hippocampal primary cultures (A), and HCT116 cells expressing (B) or lacking (C) p53, were fractionated (cytoplasm and nucleus), pre-treated for 25 min at 30°C in phosphatase buffer supplemented with protein phosphatase 2A (PP2A) or with protein phosphatase 2A and okadaic acid (OA), subjected to immunoprecipitation (IP) with eEF-2antibody or with control IgG, and analyzed by immunoblotting with a 14-3-3 antibody. β-actin and hnRNP were used as cytoplasmic and nuclear markers (bottom). Data are representative of four independent experiments. D. Hippocampal neurons were treated with 50 nM Leptomycin B. After 3 or 6 h, neurons were collected and separated into cytoplasmic and nuclear fractions, and analyzed by SDS-PAGE followed by immunoblot. β-actin and hnRNP were used as cytoplasmic and nuclear markers.E. Optical density of the ratio nucleus/cytoplasm total eEF-2bands. Values are the mean and SEM of four experiments. *p<0.05, ***p<0.001 vs. control. F. Optical density of the ratio nucleus/cytoplasm phosphorylated eEF-2 bands.