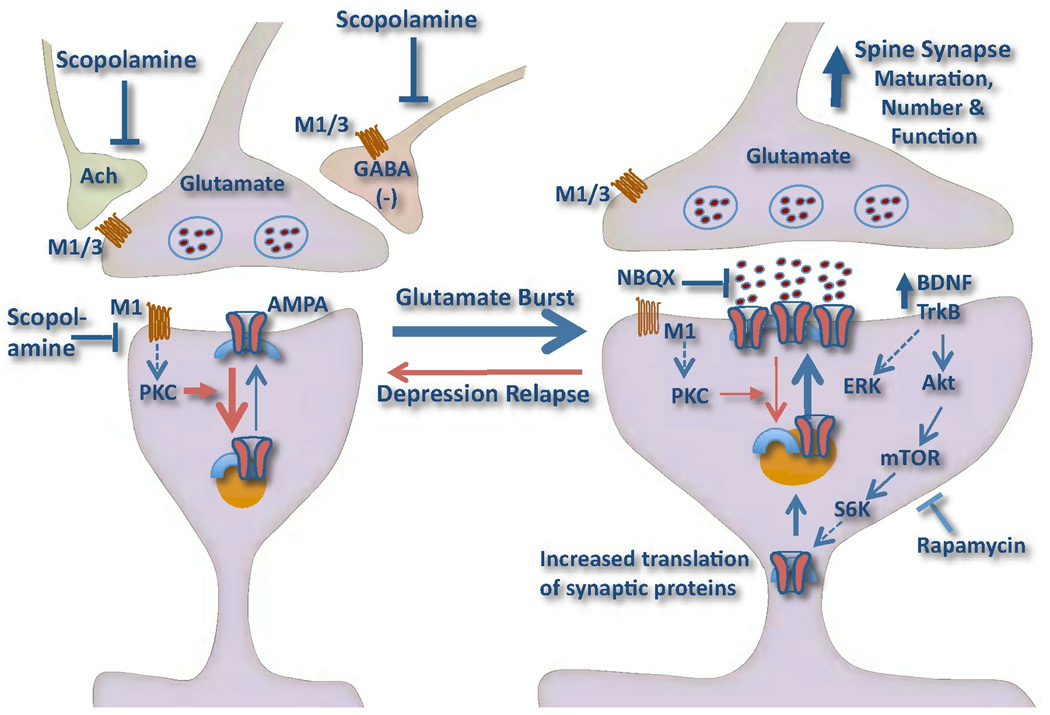
Figure 5. Model depicting the cellular mechanisms for the synaptogenic actions of scopolamine.
Scopolamine rapidly increases the number, maturation, and function of spine synapses in pyramidal neurons in the medial PFC. This could occur via increased glutamate transmission, or a glutamate burst resulting in a long-term potentiation (LTP) like synaptogenic effect. The induction of glutamate could occur via blockade of muscarinic receptors located on GABAergic interneurons, acetylcholine (Ach) terminals, or even glutamate terminals. In addition, postsynaptic muscarinic receptors that couple to protein kinase C (PKC) can produce long-term depression (LTD), and blockade of these receptors could enhance the synaptogenic response to glutamate. Scopolamine also increases mTORC1-p70S6K (S6K) signaling resulting in increased translation of synaptic proteins, including AMPA receptors, which are required for the expansion and stabilization of spines. The antidepressant behavioral responses to scopolamine require activation of the mTORC1 pathway (demonstrated by blockade with rapamycin). The actions of ketamine, another rapid acting antidepressant, require BDNF-TrkB-Akt signaling, and we hypothesize that scopolamine-induction of mTORC1 signaling also requires release of this neurotrophic factor, although this has not yet been tested. The model also shows that depression relapse, as well as stress, are associated with a reduction in spine synapses, due to failure of synaptic homeostasis. This could result from genetic mutations or environmental factors such as sustained stress, and decreased expression of BDNF.