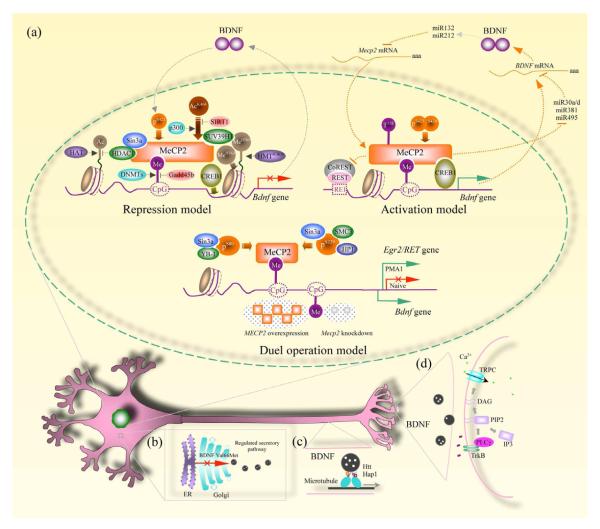
Figure 1.
BDNF deregulation in multiple regions of a neuron. a, Three models depict how MeCP2 regulate Bdnf transcription. For abbreviations and detailed description, see the text. b, BDNF is synthesized in the endoplasmic reticulum (ER) and transferred to the Golgi apparatus for proper folding. With the assistance of several proteins in the Golgi, BDNF is packaged into large dense core vesicles and targeted to the regulated secretory pathway. The Val66Met polymorphism with a valine substitution for methionine, results in the failure of proper BDNF maturation through this pathway. c, Huntingtin (Htt) and Huntingtin-associated protein 1 (Hap1) are involved in anterograde axonal transport of BDNF. Lack of these two proteins in Mecp2 knockout mice prevents BDNF from being targeted to synaptic terminals. d, BDNF released from presynaptic terminals binds to postsynaptic TrkB (tropomyosin-related kinase B) receptors and triggers their autophosphorylation. PLC is recruited to a docking site and breaks down phosphatidylinositol 4,5 bisphosphate (PIP2) into diacylglycerol (DAG) and inositol trisphosphate (IP3). DAG triggers activation of canonical transient receptor potential (TRPC) channels, resulting in membrane depolarization and Ca2+ influx. The amplitude of these postsynaptic membrane currents and Ca2+ signals are indirect estimators of the amount of BDNF released from presynaptic terminals.