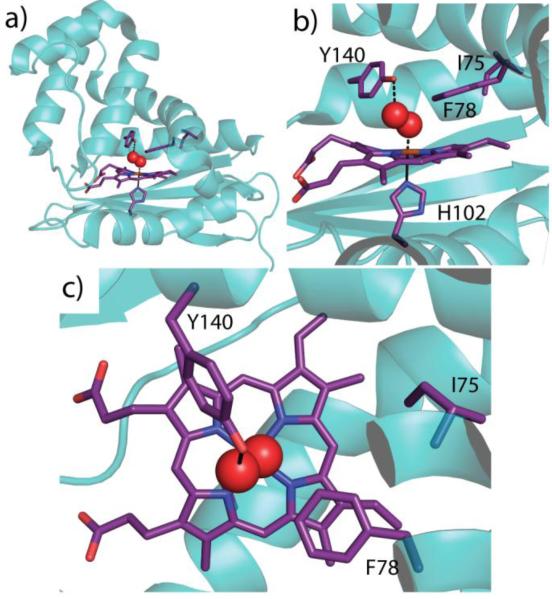
Figure 1.
Wild type Tt H-NOX (PDB ID 1U55, Reference [16]). a) Structure showing the heme and key amino acid residues shown in purple (shown in darker bond lines; amino acid residues numbered) with coordinating oxygen molecule in red spheres. b) Heme is coordinated by H102 with O2 bound above heme and stabilized by a hydrogen bond with Y140. F78 forms an off-set π-stack with the heme and I75 sits slightly further back in the heme pocket. c) Top-view of the heme pocket illustrating F78 stacked above the porphyrin