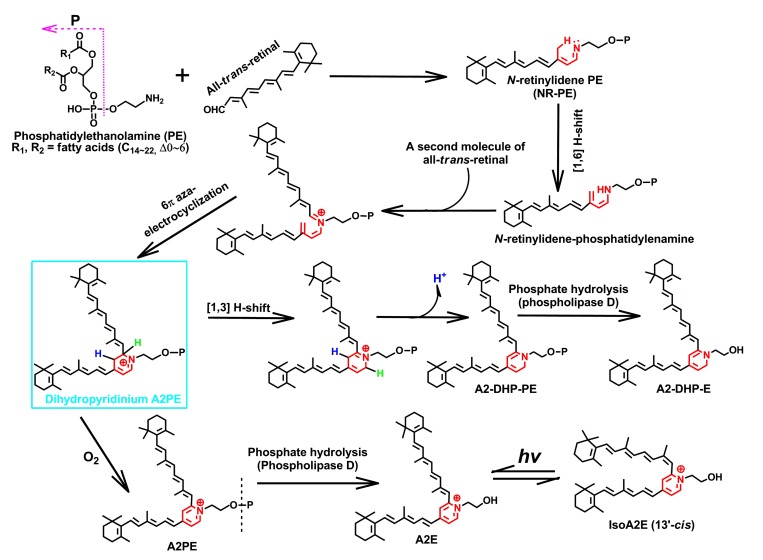
Fig. 4.
Biosynthetic pathways of N-retinylidene-N-retinyl-ethanolamine (A2E) and N-retinylidene-N-retinyl-dihydropyridine-phosphatidylethanolamine (A2-DHP-PE)
These two bis-retinoids form from a proposed uniform intermediate, dihydropyridinium N-retinylidene-N-retinyl-phosphatidylethanolamine (A2PE). The latter is enclosed by a rectangle and is not detectable in the eye and biomimetic chemical reactions, probably resulting from the facile auto-oxidation of a unique dihydropyridinium ring, from which one or double particular hydrogen is eliminated with the electronic rearrangements. Hydrogens in blue and green represent the active atoms involved in the shifting and eliminating processes in the biosynthesis of these two heterocyclic fluorophores (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)