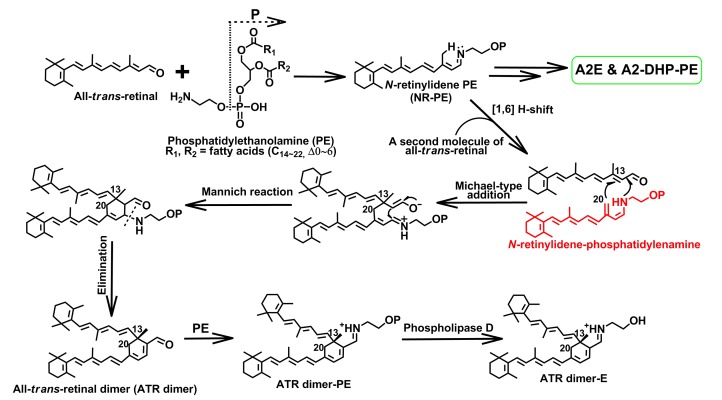
Fig. 6.
Biosynthetic pathways of all-trans-retinal (ATR) dimer, ATR dimer-E, and ATR dimer-PE
It is thought that ATR dimer series, N-retinylidene-N-retinyl-ethanolamine (A2E) and N-retinylidene-N-retinyl-dihydropyridine-phosphatidylethanolamine (A2-DHP-PE), form from the same intermediate N-retinylidene-phosphatidylenamine (see structure in red). Also, it is interesting to know that ATR dimer-E (λ max 290, 510 nm) and saturated ATR dimer-PE (λ max 290, 510 nm) exhibited a visible absorption maximum of about 80 nm red shifted from that of ATR dimer (λ max 290, 430 nm) because of a protonated Schiff base linkage formed in the position of aldehyde moiety (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)