Abstract
CCAAT/enhancer binding protein y (C/EBPα) is a basic leucine zipper transcription factor that inhibits cell cycle progression and regulates differentiation in various cell types. C/EBPα is inactivated by mutation in acute myeloid leukemia (AML) and is considered a human tumor suppressor in AML. Although C/EBPα mutations have not been observed in malignancies other than AML, greatly diminished expression of C/EBPα occurs in numerous human epithelial cancers including lung, liver, endometrial, skin, and breast, suggesting a possible tumor suppressor function. However, direct evidence for C/EBPα as an epithelial tumor suppressor is lacking due to the absence of C/EBPα mutations in epithelial tumors and the lethal effect of C/EBPα deletion in mouse model systems. To examine the function of C/EBPα in epithelial tumor development, an epidermal-specific C/EBPα knockout mouse was generated. The epidermal-specific C/EBPα knockout mice survived and displayed no detectable abnormalities in epidermal keratinocyte proliferation, differentiation, or apoptosis, showing that C/EBPα is dispensable for normal epidermal homeostasis. In spite of this, the epidermal-specific C/EBPα knockout mice were highly susceptible to skin tumor development involving oncogenic Ras. These mice displayed decreased tumor latency and striking increases in tumor incidence, multiplicity, growth rate, and the rate of malignant progression. Mice hemizygous for C/EBPα displayed an intermediate-enhanced tumor phenotype. Our results suggest that decreased expression of C/EBPα contributes to deregulation of tumor cell proliferation. C/EBPα had been proposed to block cell cycle progression through inhibition of E2F activity. We observed that C/EBPα blocked Ras-induced and epidermal growth factor-induced E2F activity in keratinocytes and also blocked Ras-induced cell transformation and cell cycle progression. Our study shows that C/EBPα is dispensable for epidermal homeostasis and provides genetic evidence that C/EBPα is a suppressor of epithelial tumorigenesis.
Introduction
CCAAT/enhancer binding protein α (C/EBPα) is a basic leucine zipper transcription factor and has a role in energy metabolism, differentiation, and mitotic growth arrest (1). Forced expression of C/EBPα results in the inhibition of cell cycle progression in most cell types, including those with activated oncogenes and inactivated tumor suppressor genes (2, 3). C/EBPα has been reported to inhibit cell proliferation through mechanisms involving (a) regulation, stabilization, and activation of the cyclin-dependent kinase (CDK) inhibitor p21 (4, 5); (b) direct inhibition of CDK4 and CDK2 activity (6); (c) interaction with Rb family members (7, 8); (d) interaction with and repression of E2F-mediated transcription activity (9, 10); and (e) interaction with an SWI/SNF complex (11). Whether all of these possible mechanisms are operative in all cells or whether certain cells use a specific subset of C/EBPα inhibitory mechanisms is not known (12).
C/EBPα is inactivated through specific somatic mutations in ~10% of acute myeloid leukemia (AML) patients (13, 14), and these studies along with work showing that C/EBPα is required for granulopoiesis in C/EBPα mutant mice (15) provide compelling evidence that C/EBPα is a tumor suppressor in AML. Although C/EBPα mutations have not been observed in malignancies other than AML, loss or greatly decreased expression occurs in numerous epithelial cancers, including lung (16), skin (17, 18), liver (19), endometrial (20), and breast cancer (21). Reexpression of C/EBPα in hepatoma cell lines (22), lung cancer lines (16), or skin squamous cell carcinoma (SCC) cell lines (18) blocks cell cycle progression. Thus, it seems that diminished expression of C/EBPα is associated with epithelial tumor development (23). However, causal or genetic evidence that C/EBPα can function as an epithelial tumor suppressor is lacking, as C/EBPα mutations have not been detected in epithelial tumors and C/EBPα-deficient mice die before or shortly after birth, presumably from altered hepatic glucose and glycogen metabolism (24). Conditional or tissue-specific knockout of C/EBPα in a tissue/organ in which tumors derived from this tissue are known to display decreased C/EBPα expression would be an ideal model system for testing whether C/EBPα has tumor suppressor function. However, this approach has also been problematic as the lung-specific loss of C/EBPα in mice results in respiratory failure at birth (25).
C/EBPα is expressed in epidermal keratinocytes of human and mouse skin (17, 26, 27). Forced expression of C/EBPα in keratinocytes inhibits cell cycle progression (28). In terms of stress responses, C/EBPα is induced in keratinocytes by a variety of DNA-damaging agents and has a role in the G1-S checkpoint in response to UVB-induced DNA damage (29). C/EBPα is expressed primarily in the suprabasal layers of the epidermis where postmitotic keratinocytes undergo differentiation and, to a lesser extent, in a subpopulation of basal keratinocytes (17, 29). The location of C/EBPα expression within the epidermis suggests that it may be involved in cell cycle exit associated with stratified squamous differentiation and/or the regulation of differentiation-specific genes. However, a role for C/EBPα in squamous differentiation remains unidentified.
The mouse skin model of multistage chemical-induced carcinogenesis is a well-defined in vivo model of epithelial neoplasia where oncogenic Ras mutations precede p53 and INK4A/ARF mutations during tumor development and progression (30, 31). Carcinogen-induced oncogenic Ras mutation is the initial critical event responsible for the development of the squamous papilloma (32). To examine the function of C/EBPα in epithelial tumor development as well as in epidermal homeostasis, we generated an epidermal-specific C/EBPα knockout mouse using the Cre-loxP recombination system. We observed that C/EBPα is dispensable for normal epidermal homeostasis; however, in spite of this, the epidermal-specific C/EBPα knockout mice are highly susceptible to Ras-induced skin tumorigenesis. Either reduced or ablated expression conferred increased susceptibility to tumorigenesis. Thus, C/EBPα functions as a tumor suppressor in epithelial tumorigenesis and our results suggest that C/EBPα suppresses Ras-mediated tumorigenesis through repression of E2F activity.
Materials and Methods
Cell culture
BALB/MK2 and BALB/MK2-Ras keratinocytes were cultured as described (18). For luciferase experiments involving Ras and the addition or omission of epidermal growth factor (EGF), cells were placed in medium deprived of growth factors (0.1% fetal bovine serum, no EGF, and 0.05 mmol/L CaCl2).
Mice
To achieve the epidermal-specific ablation, C/EBPαfl/fl mice (C57BL/6;129/SV; ref. 33) were crossed with K5Cre transgenic mice (C57BL/6;DBA), in which Cre recombinase expression is directed to the epidermis by the keratin 5 (K5) promoter (34). F1 K5Cre;C/EBPαfl/+ mice were crossed with C/EBPαfl/+ littermates to produce the five genotypes used in all experiments. C/EBPαfl/fl and K5Cre mice were genotyped by PCR as described (33, 34).
Immunoblot analysis
Immunoblot analysis was conduced as described (18) using the following antibodies: C/EBPα (1:2,000), C/EBPβ (1:2,500), or p21 (1:600) rabbit polyclonal antibodies (Santa Cruz Biotechnology) followed by horseradish peroxidase-linked donkey anti-rabbit immunoglobulin (1:2,500) from Amersham. Immunoblot analysis for detection of differentiation markers was done by incubation with involucrin (Covance), loricrin (Covance), K5 (Covance), keratin 1 (K1; Covance), or keratin 10 (K10; Covance) rabbit polyclonal antibodies at a 1:2,000 dilution followed by anti-rabbit secondary antibody at 1:2,500.
Cell proliferation and apoptosis
Mice were injected with bromodeox-yuridine (BrdUrd; 100 mg/kg body weight) and then killed 1 h later, and immunohistochemical staining was done as described (17). Apoptotic keratinocytes in the interfollicular basal epidermis were scored in H&E-stained sections and scored positive if all three of the following criteria were present: dark pyknotic nuclei, cytoplasmic eosinophilia, and absence of cellular contacts.
Tumor experiments
Wild-type, C/EBPαfl/fl, K5Cre, K5Cre;C/EBPαfl/+, and K5Cre;C/EBPαfl/fl mouse littermates (6–9 weeks old; 13 mice per group) were treated with a single application of 200 nmol 7,12-dimethylbenz(a)anthracene (DMBA; Acros) followed 1 week later with thrice weekly treatment of 5 nmol 12-O-tetradecanoylphorbol-13-acetate (TPA; LC Laboratories). All agents were applied in 200 μL acetone. Mice were killed 25 weeks after start of TPA promotion, and tumors were harvested for histologic analysis and/or DNA isolation. Two additional tumor experiments were conducted using only C/EBαfl/fl and K5Cre;C/EBPαfl/fl genotypes.
Immunohistochemical staining
Mouse skins and/or tumors were fixed in 10% neutral-buffered formalin phosphate for 24 h and embedded in paraffin. Tissue sections (5 μm) were subjected to H&E staining or specific immunohistochemistry as described (17, 18, 28
Tumor pathology
Squamous carcinomas were identified histologically as described (35) and confirmed by veterinary pathologists. Squamous carcinomas were identified based on the following criteria: severely dysplastic to anaplastic growth, marked atypia in all cell layers, lack of differentiation patterns, and most importantly invasion through the muscle layer. Tumors that exhibited these characteristics but that did not penetrate through the muscle layer were classified as carcinomas in situ.
Reporter assays
BALB/MK2 keratinocytes at 25% to 40% confluence were transfected in 12-well plates using TransFast Transfection Reagent (Promega). Cells were transfected in serum-free medium with 200 ng E2F1 promoter reporter construct or E2F mutant promoter reporter (Masa-Aki Ikeda, Tokyo Medical and Dental University, Tokyo, Japan; ref. 36) with or without the following constructs: E2F1 in pcDNA1 (37), DP1 in pCMV (38), rat C/EBPα (39) or C/EBPβ (28) in pcDNA3.1, or Ha-Ras (12V) in pcDNA3 (40). The total amount of DNA among all groups was kept constant by using empty pcDNA3.1 (Promega). For the C/EBP-responsive promoter reporter assays, cells were transfected similarly as above with 200 ng of MGF82 promoter reporter construct (41) with or without 100 ng of C/EBPα or C/EBPβ. All assays were harvested between 24 and 40 h after transfection.
Colony formation assay and NIH3T3 focus assay
BALB/MK2-Ras cells were transfected with pcDNA3 or C/EBPα, and 48 h later, the cells were trypsinized and replated at 5 × 105 per p60 dish in selection medium containing 300 μg/mL G418. NIH3T3 focus assay was conducted as described (42).
Results
C/EBPα expression is ablated in epidermis, hair follicles, and sebaceous glands of K5Cre;C/EBPαfl/fl mice
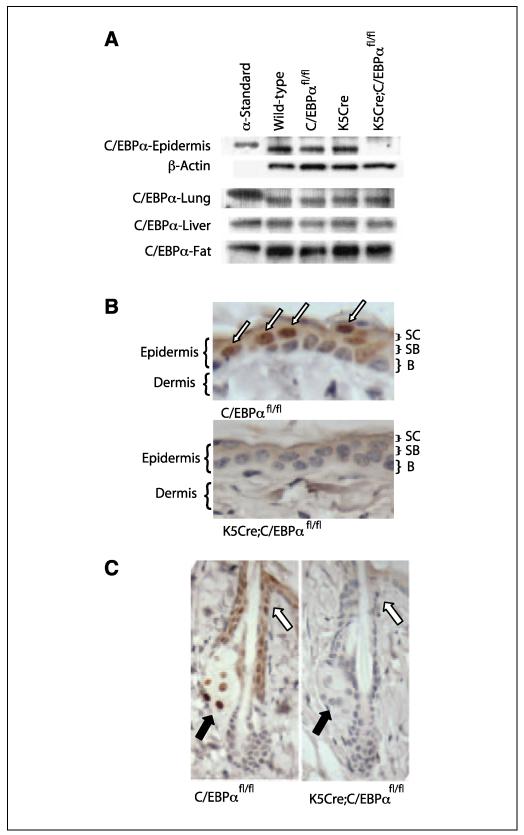
To achieve epidermal-specific ablation of C/EBPα, C/EBPafl/fl mice (33) were crossed with K5Cre transgenic mice in which Cre recombinase expression is directed to the epidermis and other stratified epithelia by the K5 promoter (34). Immunoblot analysis of epidermal lysates prepared from wild-type, C/EBPαfl/fl, K5Cre, and K5Cre;C/EBPαfl/fl mice was conducted (Fig. 1A). C/EBPα protein was not detectable in the epidermal lysates prepared from K5Cre;C/EBPαf/fl mice, although it was expressed at normal levels in the three other genotypes. To document the specificity of the ablation of C/EBPα in epidermis, we examined C/EBPα protein levels in liver, lung, and fat, three tissues known to express relatively high levels of C/EBPα (Fig. 1A). Immunoblot analysis revealed that C/EBPα was expressed at normal levels in all three tissues of all four genotypes. To examine the efficiency and location of Cre-induced recombination within the epidermis, we conducted immunohistochemical staining. In C/EBPαfl/fl mouse skin, C/EBPα staining was observed in the nuclei of interfollicular epidermal basal and suprabasal keratinocytes (Fig. 1B) as well as in hair follicles and sebaceous gland cells (Fig. 1C). In contrast, C/EBPα was not detected in any of the above structures in K5Cre;C/EBPαfl/fl mice (Fig. 1B and C), reflecting the fact that epithelial cells of the epidermis and its appendages are all derived from a common pluripotent K5-expressing stem cell (43). Epidermal stem cells are considered to be the target precursor cells for skin tumor development (44), and our results indicate that C/EBPα is ablated in these cells.
Figure 1.
C/EBPα is not expressed in the epidermis, hair follicle, and sebaceous gland of K5Cre/C/EBPαfl/fl mice. A, immunoblot analysis of C/EBPα. B, immunohistochemical staining for C/EBPα in epidermis. SC, stratum corneum; SB, suprabasal layer; B, basal layer. Arrows, nuclear C/EBPα staining C, immunohistochemical staining for C/EBPα. Black arrows, sebaceous glands; white arrows, infundibulum area of the hair follicle.
Ablation of C/EBPα in the epidermis has no effect on epidermal homeostasis
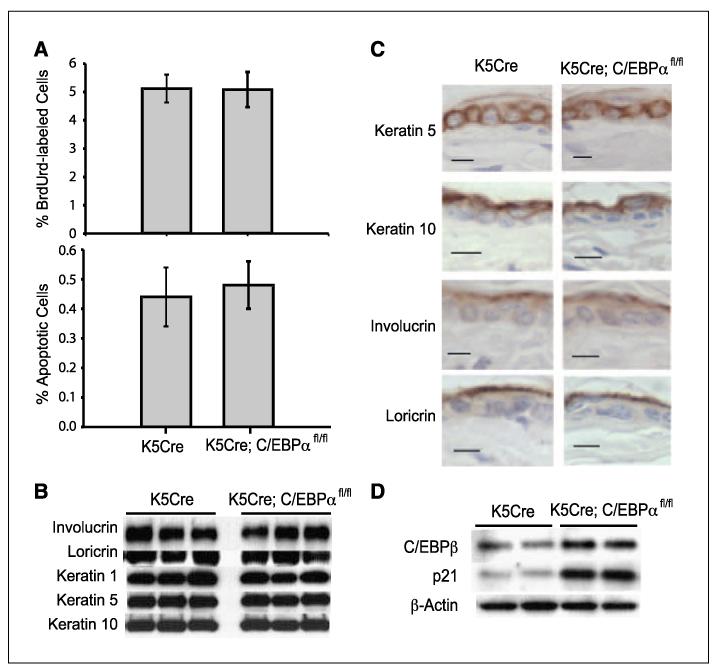
Mice with an epidermal-specific ablation of C/EBPα were born at normal Mendelian frequency. These mice did not display a visible phenotype and were grossly indistinguishable from control mice. To determine whether the loss of C/EBPα in the epidermis has any effect on epidermal homeostasis, we examined epidermal keratinocyte proliferation, apoptosis, and squamous differentiation. Surprisingly, K5Cre;C/EBPαfl/fl mice did not display any detectable alterations in epidermal keratinocyte proliferation as determined by epidermal thickness, number of nucleated cell layers (data not shown), and the number of BrdUrd-positive S-phase cells (Fig. 2A) when compared with control mice. Similarly, there were no differences in the number of apoptotic keratinocytes between control and K5Cre;C/EBPαfl/fl mice (Fig. 2A). To determine whether the loss of C/EBPα expression results in alterations in epidermal stratified squamous differentiation, we examined the expression of K5, K10, K1, involucrin, and loricrin. K5 is expressed in the basal layer keratinocytes, whereas K10 and K1 are first expressed in the transition from the basal to spinous layer, and involucrin and loricrin are expressed later in the differentiation program. Immunoblot analysis revealed that all of these markers were expressed at normal levels in the absence of epidermal C/EBPα (Fig. 2B). Immunohistochemical staining of the epidermis showed that the spatial expression of these markers was also normal in the K5Cre;C/EBPαfl/fl mice (Fig. 2C). Collectively, these results indicate that the ablation of C/EBPα in the epidermis does not alter epidermal keratinocyte proliferation, squamous differentiation, or apoptosis, showing that C/EBPα is dispensable for normal epidermal homeostasis.
Figure 2.
C/EBPα is dispensable for normal epidermal homeostasis. A, percentage BrdUrd-positive S-phase keratinocytes (top) and percentage apoptotic keratinocytes in the interfollicular basal epidermis (bottom). Columns, mean (n = 5 mice/group); bars, SE.B, immunoblot analysis of various markers of differentiation. C, immunostaining for various markers of squamous differentiation. Bar, 10 μm. D, immunoblot analysis of epidermal C/EBPβ and p21.
C/EBPβ and p21 are up-regulated in C/EBPα-deficient epidermis
The lack of effect of C/EBPα deficiency on epidermal proliferation and differentiation was unexpected and could be due to the compensatory up-regulation of genes with similar functions. C/EBPβ is expressed in the epidermis and is involved in squamous differentiation (42, 45). Therefore, we examined C/EBPβ protein levels in K5Cre;C/EBPαfl/fl epidermis. As shown in Fig. 2D, C/EBPβ was up-regulated ~2-fold in C/EBPα-deficient epidermis compared with control epidermis. The CDK inhibitor p21, a regulator of the G1- to S-phase transition in the cell cycle, was also up-regulated in the K5Cre;C/EBPαfl/fl epidermis. Increased expression of C/EBPβ and p21 may compensate for the loss of C/EBPα and potentially mask the role of C/EBPα in keratinocyte differentiation and proliferation.
Loss of C/EBPα in the epidermis results in increased susceptibility to Ras-induced skin tumorigenesis
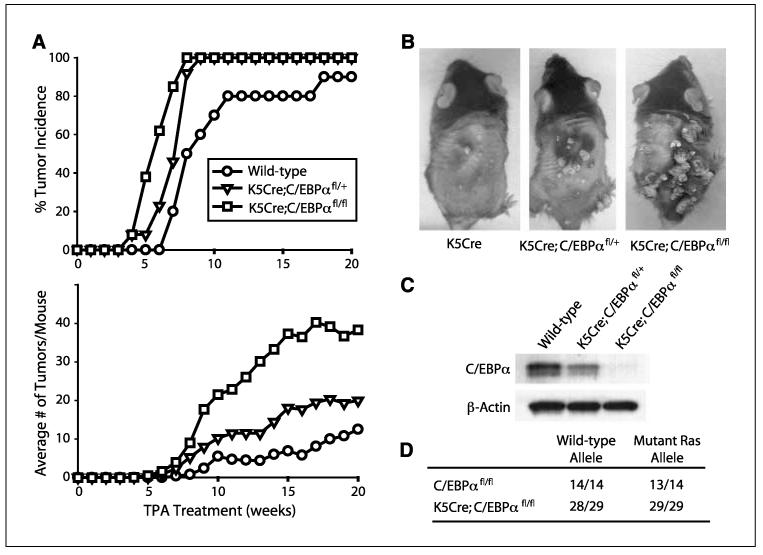
The loss of C/EBPα in the epidermis and presumably in the epidermal stem cell compartment is not sufficient in itself for skin tumor development, as untreated K5Cre;C/EBPαfl/fl mice held for 1 year did not develop any skin tumors. These results indicate that additional events are required for skin tumor development. The mouse skin model of multistage carcinogenesis involves treatment of mouse skin with DMBA followed by weekly TPA treatments and results in the production of squamous papillomas, the majority of which (>95%) contain an A→T182 transversion in Ha-Ras (32). To determine whether K5Cre;C/EBPαfl/fl mice have an altered susceptibility to tumorigenesis involving oncogenic Ras, we subjected mice to a DMBA/TPA two-stage carcinogenesis protocol. As shown in Fig. 3A, wild-type mice developed their first tumor at week 7 and at week 19 developed their maximum tumor incidence of 90% with a tumor multiplicity of ~10 tumors per mouse. C/EBPαfl/fl and K5Cre mice displayed similar tumor latency, incidence, and multiplicity as wild-type mice (data not shown). In contrast, K5Cre;C/EBPαfl/fl mice developed their first tumor at week 4, which is ~50% earlier than that of wild-type mice. All of K5Cre;C/EBPαfl/fl mice developed papillomas by week 8, and these mice developed ~40 tumors per mouse. Dramatic differences in both tumor number and size were evident in the K5Cre;C/EBPαfl/fl mice (Fig. 3B). Mice that were hemizygous for epidermal C/EBPα (K5Cre;C/EBPαfl/+) had reduced levels of C/EBPα in their epidermis (Fig. 3C) and displayed an intermediate tumor phenotype between wild-type mice and mice completely deficient in epidermal C/EBPα (Fig. 3A and B). Collectively, these results show that ablation or reduced expression of epidermal C/EBPα has a multifaceted effect on tumor development involving decreased tumor latency, increased tumor incidence, and increased tumor multiplicity in DMBA/TPA-treated mice.
Figure 3.
K5Cre;C/EBPαfl/fl mice are more susceptible to carcinogen-induced skin tumor development involving oncogenic Ras. A, tumor incidence and multiplicity (n = 13 mice/group).B, representative appearance of mice at 14 wks. C, immunoblot analysis of epidermal C/EBPα. D, activating Ras mutations were identified in codon 61 (CAA→CTA).
DMBA-induced mutation of Ha-Ras in epidermal stem cells is considered the critical event for skin tumor development (32, 44), and earlier studies showed that forced expression of C/EBPα can override the proliferative effects of oncogenic Ras in skin SCC cell lines (18). Therefore, tumors of the epidermal-specific C/EBPα knockout and control mice were examined by mutation-specific PCR to verify the presence of the DMBA-induced oncogenic Ras precursor tumor cell lesions (46). An A→T transversion in the sixty-first codon of H-Ras was present in all tumors isolated from K5Cre;C/EBPαfl/fl mice (Fig. 3D). Collectively, these results indicate that reduced or ablated expression of epidermal C/EBPα results in increased susceptibility to Ras-induced tumorigenesis.
Premalignant tumors of K5Cre;C/EBPαfl/fl mice display increased growth rate and an increased rate of malignant progression
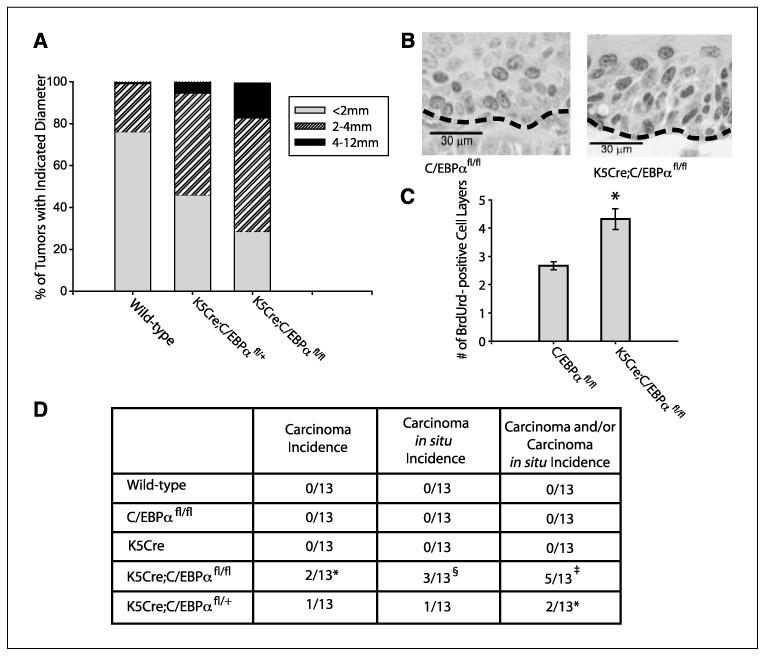
During the course of the tumor experiments, it was evident that there were striking differences in the tumor growth rate as indicated by tumor size. Grossly, these tumors were identified as papillomas. Measurement of tumor diameters showed that, at 14 weeks, only 22% of the wild-type mouse tumors were >2 mm in diameter; in contrast, 71% of the K5Cre;C/EBPαfl/fl tumors were >2 mm in diameter (Fig. 4A). The average tumor volume of K5Cre;C/EBPαfl/fl tumors was 5-fold greater than control tumor volume (data not shown). Similar to tumor multiplicity results, we observed that mice hemizygous for epidermal C/EBPα (K5Cre;C/EBPαfl/+) also displayed an intermediate tumor size phenotype between wild-type mice and mice deficient in epidermal C/EBPα (Fig. 4A). Tumors of K5Cre;C/EBPαfl/fl continued to increase in size, and the tumor experiment described in Fig. 3A was terminated at 25 weeks due to the large size of some papillomas/keratoacanthomas (>15 mm) as well as the presence of SCCs (>18 mm) in the epidermal-specific C/EBPαknockout mice. In the mouse skin model, papillomas and keratoacanthomas are considered premalignant tumors, which can progress to malignant SCCs (35). Histologic analysis revealed that the tumors were a similar combination of papillomas and keratoacanthomas in both wild-type and K5Cre;C/EBPαfl/fl mice. BrdUrd pulse-labeling studies were conducted in vivo to examine tumor cell proliferation. Histologic sections of the papillomas/keratoacanthomas revealed increased numbers of BrdUrd S-phase–positive suprabasal cell layers in K5Cre;C/EBPαfl/fl tumors compared with wild-type tumors (Fig. 4B and C). Although the increase in the number of suprabasal BrdUrd-positive cell layers contributes to the increased growth rate of the tumors, it is also known to be associated with papillomas that have a higher probability of progression to malignancy (47). No major differences in the number of apoptotic tumor cells between the genotypes were detected using terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling staining (data not shown).
Figure 4.
K5Cre;C/EBPαfl/fl mice display a significant increase in tumor growth rate and the rate of malignant progression. A, tumor diameter at 14 wks of TPA promotion. B, immunohistochemical staining for BrdUrd in tumors harvested 25 wks after start of TPA promotion.The BrdUrd-positive cells are represented by the dark staining nuclei. C, number of BrdUrd-positive cell layers in tumors. Tumors were matched in size between genotypes and harvested 25 wks after start of TPA promotion. Forty fields of view per tumor (12 tumors per genotype) were analyzed. Bars, SE. *, P < 0.01, Student’s t test. D, chart representing the carcinoma and carcinoma in situ incidence at 25 wks after TPA promotion. *, P = 0.059 for K5Cre;C/EBPαfl/fl mice versus control mice; §, P = 0.013 for K5Cre;C/EBPαfl/fl mice versus wild-type mice; ‡, P = 0.003 for K5Cre;C/EBPαfl/fl mice versus wild-type mice (Fisher’s exact test).
Progression of papillomas/keratoacanthomas to malignant squamous carcinomas is a rare and late event (>30 weeks), and C57BL6 mice are considered to be a resistant strain. Histologic examination of tumors revealed that none of the wild-type, K5Cre, or C/EBPαfl/fl mice developed SCCs or carcinoma in situ (total of 39 control mice; 13 mice per group; Fig. 4D). In contrast, 2 of 13 K5Cre;C/EBPαfl/fl mice developed SCC and both of these mice displayed two SCCs each. In addition, 3 of 13 of these mice displayed carcinoma in situ. One of 13 hemizygous mice for epidermal C/EBPα (K5Cre;C/EBPαfl/+) developed a SCC and 1 developed a carcinoma in situ (Fig. 4D). All SCCs were highly dysplastic and displayed malignant invasion into the panniculus muscle. In another smaller tumor study containing only two genotypes (K5Cre;C/EBPαfl/fl and C/EBPαfl/fl mice; n = 6/group) that was carried out for 30 weeks, we observed no SCCs or carcinoma in situ in C/EBPαfl/fl mice, whereas five of six K5Cre;C/EBPαfl/fl mice displayed at least one SCC or carcinoma in situ (two of six mice developed SCC). Collectively, these findings demonstrate that reduced expression of epidermal C/EBPα results in squamous papillomas with an increased tumor growth rate and increased rate of malignant progression.
C/EBPα blocks Ras-induced transformation, E2F activity, and cell cycle progression
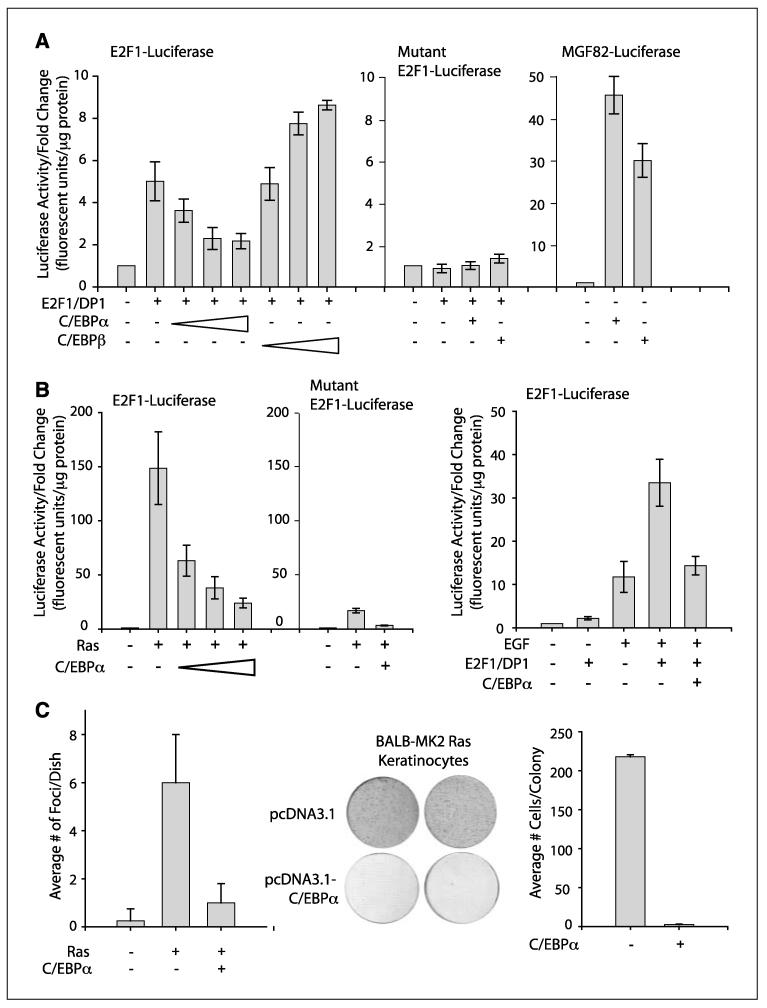
C/EBPα has been reported to inhibit cell proliferation in some cell types through the direct repression of E2F-mediated transcription (9, 10). To determine whether C/EBPα can inhibit E2F1-mediated transcription in keratinocytes, we conducted transient transfection studies in BALB/MK2 keratinocytes using E2F1 and an E2F1 promoter/reporter (36). E2F1 is an important mediator of the G1- to S-phase transition and is autoregulated at the transcriptional level during the G1 to S transition (36). Transfection of keratinocytes with E2F1/DP1 stimulated the E2F1 promoter (Fig. 5A). As shown in Fig. 5A, C/EBPα inhibited the ability of E2F1/DP1 to stimulate the E2F1 promoter in a dose-dependent manner. In contrast, C/EBPβ, a related member of the C/EBP family, did not inhibit the ability of E2F1/DP1 to stimulate the E2F1 promoter, indicating that the inhibitory effect of C/EBPα is isoform specific. Mutational inactivation of the E2F sites in the E2F1 promoter abolished the ability of E2F1/DP1 to stimulate the promoter/reporter as well as the inhibitory activity of C/EBPα (Fig. 5A). In contrast to its inhibitory action on the E2F1 promoter reporter, C/EBPα potently stimulated MGF82, a well-characterized C/EBP promoter reporter (Fig. 5A). We conducted studies to determine whether C/EBPα could inhibit oncogenic Ras-induced E2F1 promoter reporter activity. As shown in Fig. 5B, oncogenic Ras potently stimulated the E2F1 promoter and cotransfection of C/EBPα with Ras blocked the ability of Ras to stimulate the E2F1 promoter. Control experiments with the E2F1 mutant construct showed diminished Ras-induced E2F activity. EGF is a potent epithelial cell mitogen and stimulates endogenous Ras through a well-characterized EGF receptor-dependent pathway, a pathway deregulated in many epithelial tumors. As shown in Fig. 5B, C/EBPα can also inhibit EGF-induced E2F1 activity. Collectively, these results indicate that C/EBPα can inhibit E2F1/DP1 as well as Ras- and EGF-induced E2F activity in keratinocytes.
Figure 5.
C/EBPα inhibits Ras-induced E2F transcription activity, transformation, and cell cycle progression. A, reporter assay using the E2F1 promoter/reporter (left), E2F1 promoter reporter with mutant E2F sites (middle), or MGF82, a C/EBP-responsive promoter reporter (right). BALB/MK2 cells were transfected with 5 ng E2F1 and 5 ng DP1. Increasing amounts of C/EBPα or C/EBPβ were transfected (10, 30, and 100 ng) or 100 ng when one amount was used. B, reporter assay using E2F1 promoter reporter and cotransfection with 5 ng Ras (left) or with EGF treatment (right). Following transfection, cells were maintained in growth factor–depleted medium for 24 h. For EGF studies, 4 ng/mL EGF was added to cells in growth factor/serum-deprived medium following the 24 h in growth-factor depleted medium and cells were harvested 16 h after the addition of EGF. All luciferase reporter assays done in triplicate. Data are representative of at least three independent experiments. Bars, SD. C, left, C/EBPα inhibits Ras-induced transformation of NIH3T3 cells. NIH-3T3 cells were transfected with 10 μg Ras and 5 μg C/EBPα as indicated. Columns, average of four dishes per group; bars, SD. Middle and right, C/EBPα inhibits proliferation of BALB/MK2-Ras keratinocytes. Keratinocytes were transfected with 2 μg empty pcDNA3.1 or C/EBPα. Cells were fixed and stained with crystal violet at 7 d after start of G418 selection.
Next, we examined the effect of C/EBPα on Ras-induced transformation. As shown in Fig. 5C, C/EBPα blocked Ras-induced transformation of NIH3T3 cells. Similar to the Ras mutation detected in the DMBA-induced skin papillomas, BALB/MK2-Ras keratinocytes also contain endogenous oncogenic Ras with an A→T transversion in codon 61. As shown in Fig. 5C, forced expression of C/EBPα blocked cell cycle progression of BALB/MK2-Ras keratinocytes as determined by a colony formation assay. Collectively, these above results suggest that C/EBPα suppresses Ras-mediated tumorigenesis through repression of E2F activity.
Discussion
The discovery of loss-of-function mutations in C/EBPaα in human AML (13, 14) as well as seminal observations in genetically modified C/EBPα mutant mice involving hematopoiesis (10, 15) have implicated C/EBPα as a tumor suppressor in AML. Thus far, C/EBPα mutations have not been detected in epithelial tumors; however, decreased expression of C/EBPα has been reported in numerous human and mouse epithelial tumors (23). Although decreased expression of C/EBPα is consistent with a tumor suppressor function, it has not been possible to distinguish whether decreased C/EBPα expression is a cause or consequence of epithelial tumor development. Our study provides the first genetic evidence that C/EBPα has tumor suppressor activity in an epithelial tissue. Our results show that either reduced or abrogated expression of C/EBPα is permissive for Ras-induced epithelial tumorigenesis in the mouse skin tumorigenesis model. Deletion of C/EBPα in the epidermis produced a profound and multifaceted effect on carcinogen-induced tumor development, as tumor incidence, tumor multiplicity, tumor growth rate, and the rate of malignant progression were all substantially increased. These results lend credence to the functional importance of the observed decreased C/EBPα expression in skin carcinomas (17, 18) and could have important implications for other epithelial cancers, including liver, lung, breast, and endometrial, where C/EBPα expression is absent or greatly diminished (16, 18–21).
C/EBPα and epidermal homeostasis
Our results indicate that C/EBPα expression is abrogated in the epidermal stem cells of K5Cre;C/EBPαfl/fl mice, as C/EBPα is no longer expressed in the epidermis and epidermal appendages, which are all derived from the pluripotent epidermal stem cells (43). The ablation of C/EBPα in epidermis had no effect on normal epidermal homeostasis, as epidermal keratinocyte proliferation, differentiation, and apoptosis were not altered. This is particularly surprising in light of the relatively high level of C/EBPα in epidermal keratinocytes (17) as well as the potent antimitotic effect of forced C/EBPα expression in isolated keratinocytes (28). C/EBPβ, another member of the C/EBP family, is coexpressed with C/EBPα within keratinocytes of the epidermis (17, 26). C/EBPβ has a role in the early stages of squamous differentiation, and forced expression of C/EBPβ in keratinocytes blocks cell cycle progression (28). Our finding that C/EBPβ is up-regulated in the epidermis of C/EBPα-deficient mice suggests that C/EBPβ may compensate for the lack of C/EBPα and thereby mask a phenotype and function of C/EBPα in epidermal homeostasis. In support of this notion are studies showing that C/EBPβ can partially compensate for the loss of C/EBPα when C/EBPβ is knocked in to the C/EBPα locus (48). Future studies in our laboratory involving the generation and utilization of compound knockout of C/EBPα and C/EBPβ in the epidermis will address this important issue. Other compensatory responses in C/EBPα-deficient epidermis could involve the observed up-regulation of p21 levels. p21, a CDK inhibitor and member of the Cip/Kip family, is a multifunction protein in epidermis where it has a role in the regulation of cellular proliferation and differentiation (49). Both C/EBPα and p21 inhibit cell cycle progression by inhibiting the G1- to S-phase transition. In the absence of C/EBPα, an increase in p21 may prevent a hyperproliferative epidermal phenotype and thus contribute to the apparent epidermal homeostasis in the C/EBPα mutant epidermis.
C/EBPα and Ras-induced tumor development
We observed that the loss of C/EBPα in the epidermis is not sufficient in itself for skin tumor development, indicating that additional events are required for skin tumor development. The mouse skin tumorigenesis model is a well-characterized model of epithelial tumorigenesis in which DMBA-induced mutations of Ras in epidermal stem cells is a stochastic event and is considered to be the critical oncogenic lesion in the development of the premalignant squamous papilloma (30, 31, 50). Our results show that C/EBPα is a tumor suppressor in this in vivo epithelial tumorigenesis model. All tumors examined from mice deficient in epidermal C/EBPα displayed oncogenic Ras mutations, emphasizing the underlying relevance of oncogenic Ras in the development of C/EBα-deficient tumors. The increase in tumor multiplicity (~4-fold) in mice lacking epidermal C/EBPα suggests the possibility that greater numbers of Ras tumor precursor cells were capable of clonally expanding to produce premalignant tumors. The notion that the loss of C/EBPα augments Ras-induced clonal expansion is supported by the observed increase in tumor growth rate in C/EBPα-deficient tumors and by our results showing that C/EBPα can inhibit Ras-induced transformation of NIH3T3 cells and block Ras-induced E2F activity. Additional support comes from previous studies showing that forced expression of C/EBPα inhibits cell cycle progression in cells containing activated Ras (3, 9, 18).
C/EBPα is highly induced by a variety of DNA-damaging agents in keratinocytes and has a role in the G1 checkpoint in response to UVB-induced DNA damage (29). It is possible that increased tumor multiplicity in the C/EBPα epidermal-specific knockout mice is due to a diminished G1 checkpoint in response to DMBA-induced DNA damage. A diminished G1 checkpoint could increase the numbers of initiated oncogenic Ras containing tumor precursor cells available for clonal expansion. Thus, C/EBPα ablation may have dual effects on the early stages of tumor development by increasing the number of initiated oncogenic Ras cells and augmenting Ras-induced clonal expansion.
Role of C/EBPα in tumor growth and malignant progression
Most human cancer involves alterations in the cyclin D-CDK4,6/INK4A/Rb/E2F pathway. Perturbation of the “Rb” pathway results in uncontrolled cell proliferation and often involves the functional inactivation of Rb by phosphorylation due to either the activation of Ras, overexpression of D cyclins or CDKs, or inactivation of INK4A (51). Significantly, C/EBPα has been proposed to inhibit cell cycle progression through its interaction with several proteins in this critical pathway, including p21 (4), CDK4 (6), members of the Rb family (7), and E2F proteins (9). The repression of E2F activity by C/EBPα is important in the inhibition of cell proliferation in isolated cells (9) as well as in vivo, as mice expressing mutant forms of C/EBPα defective in the repression of E2F display abnormalities in cell proliferation and differentiation (10). Our finding that C/EBPα can inhibit oncogenic Ras-induced E2F activity in keratino-cytes is consistent with the E2F repression model, although it does not rule out other possibilities. E2F has been shown to cooperate with Ras to induce transformation of mouse embryonic fibroblasts (52), and various E2Fs cooperate with Ras in epithelial tumorigenesis (53, 54). Moreover, cyclin D1 or CDK4 deficiency results in decreased Ras-induced tumorigenesis (55, 56), whereas increased CDK4 activity increases tumor susceptibility (57). K5-CDK4 transgenic mice display a similar tumor phenotype to C/EBPα epidermal-specific knockout mice, as these mice are susceptible to carcinogen-induced skin tumorigenesis involving Ras and display increased tumor size, increased numbers of BrdUrd-positive tumor cells, and increased malignant progression (57). Our results suggest that the loss of C/EBPα cooperates with oncogenic Ras to contribute to the dysregulation of the Rb pathway via derepression of E2F, resulting in an increased tumor growth rate in C/EBPα-deficient tumors. In the mouse skin model, papillomas are considered premalignant lesions that progress toward SCC formation at different rates (35). The increased proliferative rate in C/EBPα-deficient premalignant lesions coupled with a diminished C/EBPα-regulated G1 checkpoint response would likely contribute to the acquisition of additional mutations and enhance malignant progression.
It is informative to compare the tumor phenotypes of epidermal-specific C/EBPα knockout mice to C/EBPβ knockout mice (42). Although C/EBPα and C/EBPβ are 90% similar in their basic leucine zipper domain and are considered to bind the same DNA consensus sequence (1), they have opposite effects on skin tumor development. C/EBPβ knockout mice are completely refractory to skin tumorigenesis involving Ras, and our previous studies indicate that C/EBPβ can cooperate with Ras to induce transformation (42, 58). Thus, it is possible that increased expression of C/EBPβ in C/EBPα-deficient epidermis contributes to the enhanced tumor phenotype observed in C/EBPα-deficient mice. Similarly, C/EBPβ deficiency results in C/EBPα being the predominant form of C/EBP and this may contribute to the observed resistance to skin tumorigenesis in C/EBPβ knockout mice. Thus, it seems that these two family members have a yin-yang relationship in tumorigenesis such that removing one member disrupts the balance and has profound effects on the activity of the other.
We observed that reduced or abrogated expression of C/EBPα in epidermis has a profound effect on many aspects of tumor development but has no effect on normal epidermal differentiation and proliferation. These results are in contrast to AML where loss of C/EBPα function results in a block in the differentiation of granulocytic blasts, and this is considered a critical event in expansion of the myeloid precursor population (59). Our findings suggest that the loss of C/EBPα contributes to epidermal tumorigenesis through a mechanism that results in the deregulation of tumor cell proliferation independent of an effect on cellular differentiation. In summary, our results provide genetic evidence that C/EBPα is a tumor suppressor in epithelial tumorigenesis and suggest that C/EBPα suppresses Ras-mediated tumorigenesis through repression of E2F activity.
Acknowledgments
Grant support: National Cancer Institute grant CA46637, National Institute of Environmental Health Sciences grant ES12473, and Graduate Assistance in Areas of National Need Fellowship.
We thank Drs. David Malarkey and Mac Law for their advice and histologic analysis of the tumors, Dr. John Horowitz for the kind gift of the E2F1 and DP1 constructs, Dr. Masa-Aki Ikeda for the E2F1 and mutant E2F1 promoter reporter constructs, and Drs. Angel Ramirez and Jose L. Jorcano for the K5-Cre transgenic mice.
References
- 1.Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J. 2002;365:561–75. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Umek RM, Friedman AD, McKnight SL. CCAAT-enhancer binding protein: a component of a differentiation switch. Science. 1991;251:288–92. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- 3.Hendricks-Taylor LR, Darlington GJ. Inhibition of cell proliferation by C/EBPα occurs in many cell types, does not require the presence of p53 or Rb, and is not affected by large T-antigen. Nucleic Acids Res. 1995;23:4726–33. doi: 10.1093/nar/23.22.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Timchenko NA, Wilde M, Nakanishi M, Smith JR, Darlington GJ. CCAAT/enhancer-binding protein a (C/EBPα) inhibits cell proliferation through the p21 (WAF-1/CIP-1/SDI-1) protein. Genes Dev. 1996;10:804–15. doi: 10.1101/gad.10.7.804. [DOI] [PubMed] [Google Scholar]
- 5.Harris TE, Albrecht JH, Nakanishi M, Darlington GJ. CCAAT/enhancer-binding protein-a cooperates with p21 to inhibit cyclin-dependent kinase-2 activity and induces growth arrest independent of DNA binding. J Biol Chem. 2001;276:29200–9. doi: 10.1074/jbc.M011587200. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Iakova P, Wilde M, et al. C/EBPα arrests cell proliferation through direct inhibition of Cdk2 and Cdk4. Mol Cell. 2001;8:817–28. doi: 10.1016/s1097-2765(01)00366-5. [DOI] [PubMed] [Google Scholar]
- 7.Timchenko NA, Wilde M, Iakova P, Albrecht JH, Darlington GJ. E2F/107 and E2F/p130 complexes are regulated by C/EBPα in 3T3-L1 adipocytes. Nucleic Acids Res. 1999;27:3621–30. doi: 10.1093/nar/27.17.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timchenko NA, Wilde M, Darlington GJ. C/EBPα regulates formation of S-phase-specific E2F-p107 complexes in livers of newborn mice. Mol Cell Biol. 1999;19:2936–45. doi: 10.1128/mcb.19.4.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slomiany BA, D’Arigo KL, Kelly MM, Kurtz DT. C/EBPα inhibits cell growth via direct repression of E2F-DP-mediated transcription. Mol Cell Biol. 2000;20:5986–97. doi: 10.1128/mcb.20.16.5986-5997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porse BT, Pedersen TA, Xu X, et al. E2F repression by C/EBPα is required for adipogenesis and granulopoiesis in vivo. Cell. 2001;107:247–58. doi: 10.1016/s0092-8674(01)00516-5. [DOI] [PubMed] [Google Scholar]
- 11.Muller C, Calkhoven CF, Sha X, Leutz A. C/EBPα requires a SWI/SNF complex for proliferation arrest. J Biol Chem. 2004;279:7353–8. doi: 10.1074/jbc.M312709200. [DOI] [PubMed] [Google Scholar]
- 12.Johnson PF. Molecular stop signs: regulation of cell-cycle arrest by C/EBP transcription factors. J Cell Sci. 2005;118:2545–55. doi: 10.1242/jcs.02459. [DOI] [PubMed] [Google Scholar]
- 13.Pabst T, Mueller BU, Zhang P, et al. Dominant-negative mutations of C/EBPA, encoding CCAAT/enhancer binding protein-a, in acute myeloid leukemia. Nat Genet. 2001;27:263–70. doi: 10.1038/85820. [DOI] [PubMed] [Google Scholar]
- 14.Gombart AF, Hofmann WK, Kawano S, et al. Mutations in the gene encoding the transcription factor CCAAT/enhancer binding protein a in myelodysplastic syndromes and acute myeloid leukemias. Blood. 2002;99:1332–40. doi: 10.1182/blood.v99.4.1332. [DOI] [PubMed] [Google Scholar]
- 15.Zhang DE, Zhang P, Wang ND, Hetherington CJ, Darlington GJ, Tenen DG. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein α-deficient mice. Proc Natl Acad Sci U S A. 1997;94:569–74. doi: 10.1073/pnas.94.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halmos B, Huettner CS, Kocher O, Ferenczi K, Karp DD, Tenen DG. Down-regulation and antiproliferative role of C/EBPα in lung cancer. Cancer Res. 2002;62:528–34. [PubMed] [Google Scholar]
- 17.Oh H-S, Smart RC. Expression of CCAAT/enhancer binding protein (C/EBP) is associated with squamous differentiation in epidermis and isolated primary keratinocytes and is altered in skin neoplasms. J Invest Dermatol. 1998;110:939–45. doi: 10.1046/j.1523-1747.1998.00199.x. [DOI] [PubMed] [Google Scholar]
- 18.Shim M, Powers KL, Ewing SJ, Zhu S, Smart RC. Diminished expression of C/EBPα in skin carcinomas is linked to oncogenic Ras and reexpression of C/EBPα in carcinoma cells inhibits proliferation. Cancer Res. 2005;65:861–7. [PubMed] [Google Scholar]
- 19.Xu L, Hui L, Wang S, et al. Expression profiling suggested a regulatory role of liver-enriched transcription factors in human hepatocellular carcinoma. Cancer Res. 2001;61:3176–81. [PubMed] [Google Scholar]
- 20.Takai N, Kawamata N, Walsh CS, et al. Discovery of epigenetically masked tumor suppressor genes in endometrial cancer. Mol Cancer Res. 2005;3:261–9. doi: 10.1158/1541-7786.MCR-04-0110. [DOI] [PubMed] [Google Scholar]
- 21.Gery S, Tanosaki S, Bose S, Bose N, Vadgama J, Koeffler HP. Down-regulation and growth inhibitory role of C/EBPα in breast cancer. Clin Cancer Res. 2005;11:3184–90. doi: 10.1158/1078-0432.CCR-04-2625. [DOI] [PubMed] [Google Scholar]
- 22.Watkins PJ, Condreay JP, Huber BE, Jacobs SJ, Adams DJ. Impaired proliferation and tumorigenicity induced by CCAAT/enhancer-binding protein. Cancer Res. 1996;56:1063–7. [PubMed] [Google Scholar]
- 23.Schuster MB, Porse BT. C/EBPα: a tumour suppressor in multiple tissues? Biochim Biophys Acta. 2006;1766:88–103. doi: 10.1016/j.bbcan.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Wang ND, Finegold MJ, Bradley A, et al. Impaired energy homeostasis in C/EBPα knockout mice. Science. 1995;269:1108–12. doi: 10.1126/science.7652557. [DOI] [PubMed] [Google Scholar]
- 25.Basseres DS, Levantini E, Ji H, et al. Respiratory failure due to differentiation arrest and expansion of alveolar cells following lung-specific loss of the transcription factor C/EBPα in mice. Mol Cell Biol. 2006;26:1109–23. doi: 10.1128/MCB.26.3.1109-1123.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maytin EV, Habener JF. Transcription factors C/EBPα, C/EBPβ, and CHOP (Gadd153) expressed during the differentiation program of keratinocytes in vivo and in vitro. J Invest Dermatol. 1998;110:238–46. doi: 10.1046/j.1523-1747.1998.00123.x. [DOI] [PubMed] [Google Scholar]
- 27.Swart GWM, van Groningen JJM, van Ruissen F, Bergers M, Schalkwilk J. Transcription factor C/EBPα: novel sites of expression and cloning of the human gene. Biol Chem. 1997;378:373–9. doi: 10.1515/bchm.1997.378.5.373. [DOI] [PubMed] [Google Scholar]
- 28.Zhu S, Oh HS, Shim M, Sterneck E, Johnson PF, Smart RC. C/EBPβ modulates the early events of keratinocyte differentiation involving growth arrest and keratin 1 and keratin 10 expression. Mol Cell Biol. 1999;19:7181–90. doi: 10.1128/mcb.19.10.7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon K, Smart RC. C/EBPα is a DNA damage-inducible p53-regulated mediator of the G1 checkpoint in keratinocytes. Mol Cell Biol. 2004;24:10650–60. doi: 10.1128/MCB.24.24.10650-10660.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiGiovanni J. Multistage carcinogenesis in mouse skin. Pharmacol Ther. 1992;54:63–128. doi: 10.1016/0163-7258(92)90051-z. [DOI] [PubMed] [Google Scholar]
- 31.Balmain A, Harris CC. Carcinogenesis in mouse and human cells: parallels and paradoxes. Carcinogenesis. 2000;21:371–7. doi: 10.1093/carcin/21.3.371. [DOI] [PubMed] [Google Scholar]
- 32.Quintanilla M, Brown K, Ramsden M, Balmain A. Carcinogen specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986;322:78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- 33.Lee YH, Sauer B, Johnson PF, Gonzalez FJ. Disruption of the c/ebpα gene in adult mouse liver. Mol Cell Biol. 1997;17:6014–22. doi: 10.1128/mcb.17.10.6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramirez A, Page A, Gandarillas A, et al. A keratin K5Cre transgenic line appropriate for tissue-specific or generalized Cre-mediated recombination. Genesis. 2004;39:52–7. doi: 10.1002/gene.20025. [DOI] [PubMed] [Google Scholar]
- 35.Aldaz CM, Conti CJ, Klein-Szanto AJ, Slaga TJ. Progressive dysplasia and aneuploidy are hallmarks of mouse skin papillomas: relevance to malignancy. Proc Natl Acad Sci U S A. 1987;84:2029–32. doi: 10.1073/pnas.84.7.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson DG, Ohtani K, Nevins JR. Autoregulatory control of E2F1 expression in response to positive and negative regulators of cell cycle progression. Genes Dev. 1994;8:1514–25. doi: 10.1101/gad.8.13.1514. [DOI] [PubMed] [Google Scholar]
- 37.Cress WD, Johnson DG, Nevins JR. A genetic analysis of the E2F1 gene distinguishes regulation by Rb, p107, and adenovirus E4. Mol Cell Biol. 1993;13:6314–25. doi: 10.1128/mcb.13.10.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Helin K, Wu CL, Fattaey AR, et al. Heterodimerization of the transcription factors E2F-1 and DP-1 leads to cooperative trans-activation. Genes Dev. 1993;7:1850–61. doi: 10.1101/gad.7.10.1850. [DOI] [PubMed] [Google Scholar]
- 39.Williams SC, Cantwell CA, Johnson PF. A family of C/EBP-related proteins capable of forming covalently linked leucine zipper dimers in vitro. Genes Dev. 1991;5:1553–67. doi: 10.1101/gad.5.9.1553. [DOI] [PubMed] [Google Scholar]
- 40.Der CJ, Finkel T, Cooper GM. Biological and biochemical properties of human rasH genes mutated at codon 61. Cell. 1986;44:167–76. doi: 10.1016/0092-8674(86)90495-2. [DOI] [PubMed] [Google Scholar]
- 41.Sterneck E, Muller C, Katz S, Leuz A. Autocrine growth induced by kinase type oncogenes in myeloid cells requires AP-1 and NF-M, a myeloid specific, C/EBP-like factor. EMBO J. 1992;11:115–26. doi: 10.1002/j.1460-2075.1992.tb05034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu S, Yoon K, Sterneck E, Johnson PF, Smart RC. CCAAT/enhancer binding protein-h is a mediator of keratinocyte survival and skin tumorigenesis involving oncogenic Ras signaling. Proc Natl Acad Sci U S A. 2002;99:207–12. doi: 10.1073/pnas.012437299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–61. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- 44.Owens DM, Watt FM. Contribution of stem cells and differentiated cells to epidermal tumours. Nat Rev Cancer. 2003;3:444–51. doi: 10.1038/nrc1096. [DOI] [PubMed] [Google Scholar]
- 45.Maytin EV, Lin JC, Krishnamurthy R, et al. Keratin 10 gene expression during differentiation of mouse epidermis requires transcription factors C/EBP and AP-2. Dev Biol. 1999;216:164–81. doi: 10.1006/dbio.1999.9460. [DOI] [PubMed] [Google Scholar]
- 46.Nelson MA, Futscher BW, Kinsella T, Wymer J, Bowden GT. Detection of mutant Ha-ras genes in chemically initiated mouse skin epidermis before the development of benign tumors. Proc Natl Acad Sci U S A. 1992;89:6398–402. doi: 10.1073/pnas.89.14.6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tennenbaun T, Weiner AK, Belanger AJ, Glick AB, Hennings H, Yuspa SH. The suprabasal expression of α6β4 integrin is associated with a high risk for malignant progression in mouse skin carcinogenesis. Cancer Res. 1993;53:4803–10. [PubMed] [Google Scholar]
- 48.Chen SS, Chen JF, Johnson PF, Muppala V, Lee YH. C/EBPβ, when expressed from the C/EBPα gene locus, can functionally replace C/EBPα in liver but not in adipose tissue. Mol Cell Biol. 2000;20:7292–9. doi: 10.1128/mcb.20.19.7292-7299.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DiCunto F, Topley G, Calautti E, et al. Inhibitory function of p21Cip1/WAF1in differentiation of primary mouse keratinocytes independent of cell cycle control. Science. 1998;280:1069–72. doi: 10.1126/science.280.5366.1069. [DOI] [PubMed] [Google Scholar]
- 50.Yuspa SH. The pathogenesis of squamous cell cancer: lessons learned from studies of skin carcinogenesis— thirty-third G. H. A. Clowes Memorial Award Lecture. Cancer Res. 1994;54:1178–89. [PubMed] [Google Scholar]
- 51.Malumbres M, Barbacid M. To cycle or not to cycle: a critical decision in cancer. Nat Rev Cancer. 2001;1:222–31. doi: 10.1038/35106065. [DOI] [PubMed] [Google Scholar]
- 52.Johnson DG, Cress WD, Jakoi L, Nevins JR. Oncogenic capacity of the E2F1 gene. Proc Natl Acad Sci U S A. 1994;91:12823–7. doi: 10.1073/pnas.91.26.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pierce AM, Fisher SM, Conti CJ, Johnson DG. Deregulated expression of E2F1 induces hyperplasia and cooperates with ras in skin tumor development. Oncogene. 1998;16:1267–76. doi: 10.1038/sj.onc.1201666. [DOI] [PubMed] [Google Scholar]
- 54.Paulson QX, McArthur MJ, Johnson DG. E2F3a stimulates proliferation, p53-independent apoptosis and carcinogenesis in a transgenic mouse model. Cell Cycle. 2006;5:184–90. doi: 10.4161/cc.5.2.2307. [DOI] [PubMed] [Google Scholar]
- 55.Yu Q, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411:1017–21. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez-Puebla ML, Miliani de Marval PL, LaCava M, Moons DS, Kiyokawa H, Conti CJ. Cdk4 deficiency inhibits skin tumor development but does not affect normal keratinocyte proliferation. Am J Pathol. 2002;161:405–11. doi: 10.1016/S0002-9440(10)64196-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miliani de Marval PL, Macias E, Conti CJ, Rodriguez-Puebla ML. Enhanced malignant tumorigenesis in Cdk4 transgenic mice. Oncogene. 2004;23:1863–73. doi: 10.1038/sj.onc.1207309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sterneck E, Zhu S, Ramirez A, Jorcano JL, Smart RC. Conditional ablation of C/EBPβ demonstrates its keratinocyte-specific requirement for cell survival and mouse skin tumorigenesis. Oncogene. 2006;25:1272–6. doi: 10.1038/sj.onc.1209144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nerlov C. C/EBPα mutations in acute myeloid leukaemias. Nat Rev Cancer. 2004;4:394–400. doi: 10.1038/nrc1363. [DOI] [PubMed] [Google Scholar]