Fig. 7.
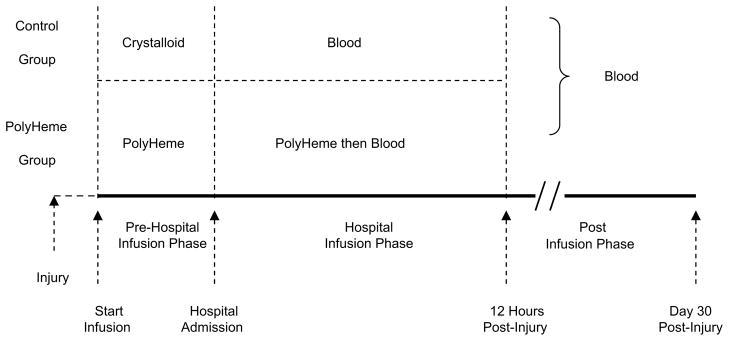
Study design. Patients were randomized (50:50) into each treatment group. PolyHeme patients received PolyHeme in the field, then PolyHeme (up to six units) for up to 12 hours, and then blood in the hospital, as needed. Control patients received crystalloid in the field, then RBC transfusion in the hospital as needed. (Adapted from Moore EE, Moore FA, Fabian TC, et al. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA Multicenter Trial. J Am Coll Surg 2009;208:1–13; with permission.)
