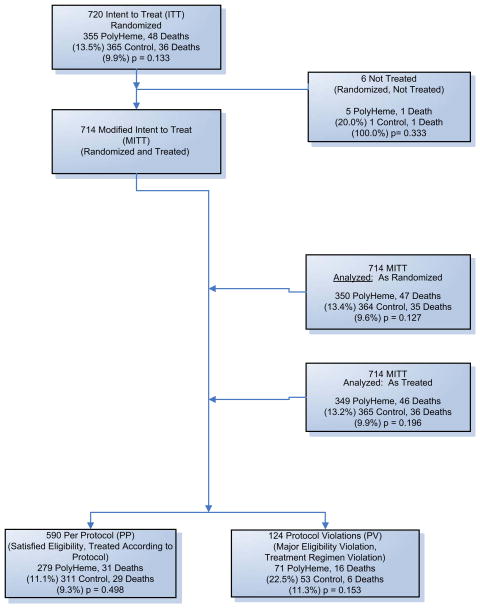
Fig. 8.
Study patient enrollment. Patients were assessed for eligibility by paramedics in the field at the scene of injury; 722 patients were enrolled in the study. Two patients had their data withheld by the local institutional review board. The Modified Intent to Treat (MITT) population comprises 720 patients. The MITT population is analyzed two ways: “As Randomized,” in which patients are analyzed in the group to which they were randomized (regardless of which treatment the patients received), and “As Treated,” in which patients are analyzed according to the treatment they actually received. Of the 720 patients, 590 satisfied all the eligibility criteria and were treated according to the protocol; 124 violated eligibility criteria or did not adhere to the treatment regimen described in the protocol. (Adapted from Moore EE, Moore FA, Fabian TC, et al. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA Multicenter Trial. J Am Coll Surg 2009;208:1–13; with permission.)