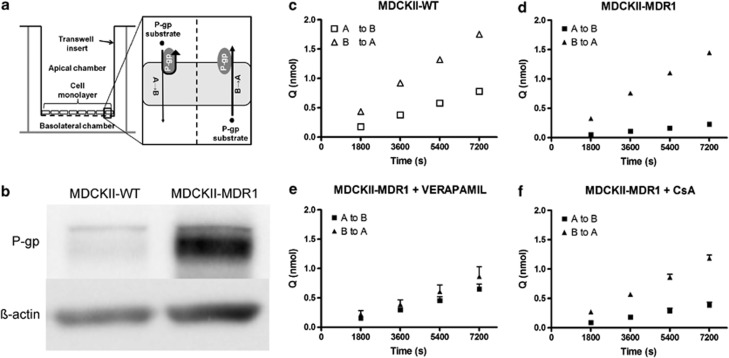
Figure 1.
In vitro bidirectional transport studies. (a) Schematic illustrating the in vitro bidirectional transport assay. MDCKII-MDR1 cells express human P-glycoprotein (P-gp) in a polarized manner at the apical membrane only, when cultured on a transwell support. When substrates of human P-gp are added to the apical chamber, their transport across the monolayer of cells is restricted by P-gp efflux, thereby reducing their apical-to-basolateral (A→B) permeability. When substrates of human P-gp are added to the basolateral chamber, P-gp does not limit their transport across the monolayer as it is not expressed at the basolateral membrane. Therefore, human P-gp substrates will have greater permeability in the basolateral-to-apical (B→A) direction than in the A→B direction, and directional efflux is attenuated by coincubation of a P-gp inhibitor. Bidirectional transport studies are also carried out in MDCKII-WT cells, which do not express human P-gp, to determine the influence of endogenous transporters, including canine P-gp, on drug permeability. (b) Representative image of western blot demonstrating over 4.5-fold greater expression of P-gp in MDCKII-MDR1 cells than MDCKII-WT cells. (c and d) Escitalopram accumulation in receiver chamber over time in MDCKII-WT and MDCKII-MDR1 cells. Escitalopram crossed the monolayer to a greater extent in the B→A direction than A→B direction in both MDCKII-WT (c) and MDCKII-MDR1 (d) cells, indicating net efflux in both cell lines. However, the magnitude of the efflux effect was substantially greater in MDCKII-MDR1 cells, highlighting a role for human P-gp. (e and f) Escitalopram accumulation in receiver chamber over time in MDCKII-MDR1 cells with coincubation of the P-gp inhibitors verapamil (e) or cyclosporin A (CsA) (f). Coincubation with either P-gp inhibitor attenuated the directional efflux of escitalopram, thereby confirming that escitalopram is a transported substrate of human P-gp (mean±SEM; n=3 in all experiments). WT, wild type.