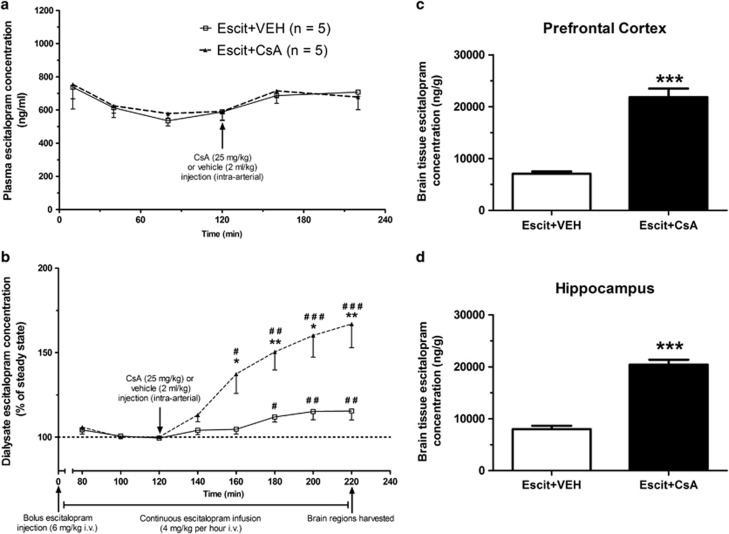
Figure 2.
In vivo pharmacokinetic studies. (a) Plasma escitalopram pharmacokinetics. There was no difference in escitalopram plasma pharmacokinetics between vehicle- and cyclosporin A (CsA)-treated animals. Steady-state plasma escitalopram levels were achieved within 120 min in both groups. Administration of vehicle or the P-glycoprotein (P-gp) inhibitor CsA resulted in an equivalent 15–20% increase in plasma escitalopram levels from steady state in both groups. (b) Dialysate escitalopram pharmacokinetics. Steady-state dialysate concentrations of escitalopram were also achieved in both groups within 120 min. Administration of vehicle resulted in a significant 17% increase in dialysate escitalopram concentration from steady-state levels, which mirrored increases observed in the plasma. Administration of the P-gp inhibitor CsA resulted in a 67% increase from steady state, which was significantly greater than that observed in the vehicle-treated group. (c and d) Brain tissue escitalopram concentrations. Escitalopram concentrations in brain tissue at termination of in vivo pharmacokinetic studies were significantly greater in CsA-treated animals than vehicle-treated animals in both the prefrontal cortex (c) and the hippocampus (d) (mean±SEM; n=5 per group for all graphs). *p<0.05; **p<0.01; ***p<0.001 between the groups; #p<0.05; ##p<0.01; ###p<0.001 compared with steady-state levels.