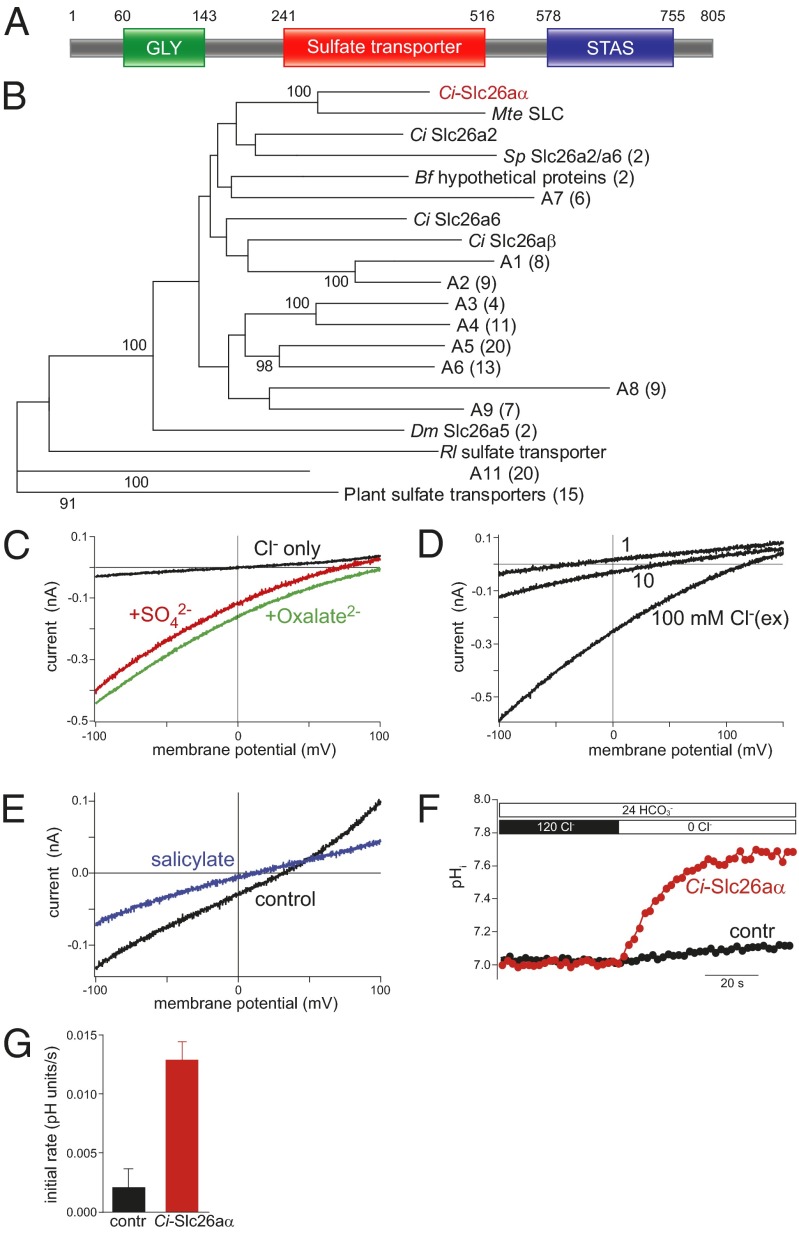
Fig. 1.
Ci-Slc26aα is an SLC26 family protein. (A) Domain structure of Ci-Slc26aα. (B) Neighbor-joining tree of 134 sulfate transporters. Bootstrap values are based on 100 replicates, and only those >90 are shown on the branches. (C) Electrogenic anion transport mediated by Ci-Slc26aα in CHO cells. Transport currents occurred in the presence of either sulfate or oxalate (10 mM, intracellular), but not when solely monovalent anions were present (10 mM intracellular and 100 mM extracellular Cl−). (D) Transport current reversal potential shifted strongly to more negative voltages as the extracellular Cl− concentration decreased, indicating coupled antiport of Cl− against the divalent anion. (E) Inhibition of sulfate transport current by 30 mM salicylate. Intracellular and extracellular Cl− concentrations were both 10 mM; intracellular and extracellular sulfate concentrations were 10 and 1 mM, respectively. (F) Ratiometric measurement of Cl−/HCO3− exchange by Ci-Slc26aα. CHO cells expressing Ci-Slc26aα (red) or lacking expression (black) were loaded with the ratiometric pH dye BCECF and superfused with HCO3−. On removal of Cl−, a rise in intracellular pH indicates Cl−/HCO3− antiport driven by the inward Cl− gradient. (G) Initial rates of pH change were 0.013 ± 0.002 s−1 (n = 12 cells) for Ci-Slc26aα cells and and 0.002 ± 0.002 s−1 (n = 9) control cells. Ci, Ciona intestinalis; Mt, Molgula tectiformis; Sp, Strongylocentrotus purpuratus; Bf, Branchiostoma floridae; Dm, Drosophila melanogaster; Rl, Rhizobium leguminosarum.