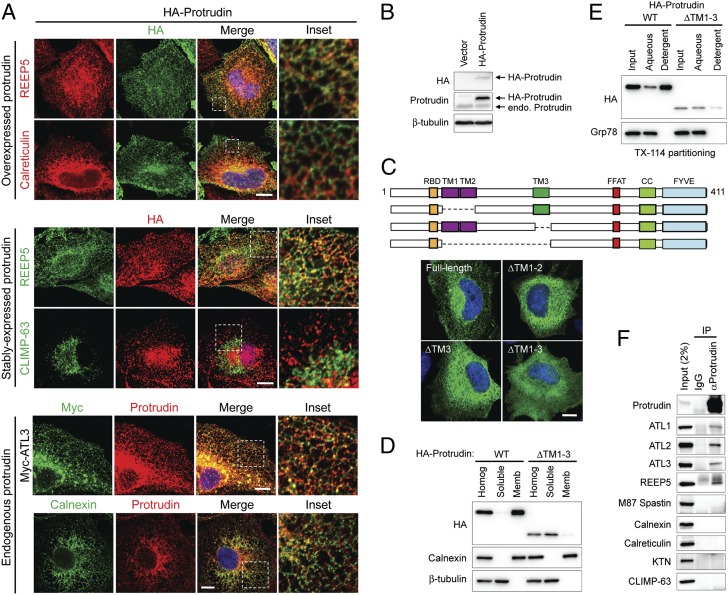
Fig. 2.
Protrudin localizes to the ER via its intramembrane domains and interacts with ER-shaping proteins. (A) From top to bottom, HeLa cells transiently expressing HA-protrudin were immunostained for endogenous REEP5 or calreticulin (red), along with HA-epitope (green). HeLa cells stably expressing HA-protrudin were immunostained for endogenous REEP5 or CLIMP-63 (green) and HA-epitope (red). Cells expressing Myc–atlastin-3 were coimmunostained for Myc-epitope (green) and endogenous protrudin (red). HeLa cells were costained for endogenous calnexin (green) and endogenous protrudin (red). Insets in the merged images (with DAPI staining) are enlarged to the right. (B) Lysates from cells stably expressing HA-protrudin were immunoblotted as shown. Endo., endogenous. (C, Upper) Schematic diagram of human protrudin and ΔTM deletion constructs. CC, coiled-coil; RBD, Rab-binding domain. (C, Lower) Cells expressing HA-tagged wild-type (WT) protrudin and the indicated deletion mutants were stained for HA-epitope (green) and DAPI (blue). (D) Wild-type and mutant protrudin proteins as in C were expressed in HEK293T cells. Homogenates (Homog) were separated into soluble and membrane (Memb) fractions and immunoblotted. (E) Membrane fractions from cells expressing wild-type and mutant protrudin proteins were phase partitioned with Triton X-114 and then immunoblotted for HA-epitope or Grp78. (F) Endogenous protrudin in HEK293T cells was immunoprecipitated (IP) with anti-protrudin antibodies or control IgG, and precipitates were immunoblotted as shown. ATL, atlastin; KTN, kinectin. (Scale bars: 10 μm.)