Figure 3.
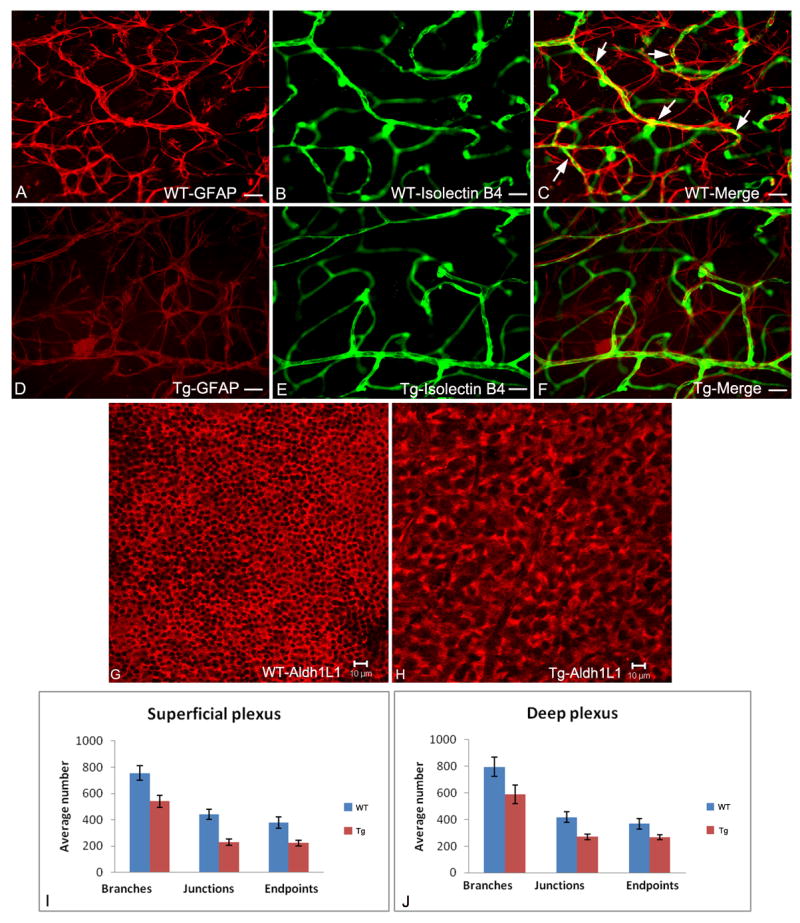
Fluorescent microscopy of retinal flat mounts from 3 week old wild type and transgenic mice with astrocytes immunolabelled with anti-GFAP (red; A, D); blood vessels immunolabelled with isolectin-B4 (green; B, E) with merged images shown in C, F. The compact stellate structure and honeycomb template of wild type astrocytes (A) is disrupted in the transgenic animals (D). The capillary plexus pattern is less dense in the transgenic (E) compared to normal (F) retina. In wild type retina (C), the astrocyte processes enwrap the blood vessels (yellow, arrows); this is not seen in the transgenic retinas (F). Confocal microscopy of retinal flat mounts from 3 week old mice with astrocytes immunolabelled with anti-Aldh1L1 (red channel) clearly shows abnormal morphology and less dense astrocytes in transgenic mice overexpressing the mutant (Nuc1) βA3/A1-crystallin (H) compared to astrocytes in normal template of the retina (G). Quantification of the superficial (I) and deep plexus (J) in wild type and transgenic mice was performed by scoring the vascular architecture using the digital analysis software image J (NIH). The major vascular parameters were scored by counting the number of branches, junctions and endpoints per 30 different microscope fields (40X) taken from the same region in multiple animals (n=3 wild type; n=5 transgenic). Binary images were created, skeletonized and analyzed using the ‘analyze skeleton plugin of image J. The retinal vasculature of transgenic animals showed a reduction in the number of branches, junctions and endpoints in the superficial (I) and deep plexus (J) compared to their wild type controls. Scale bar: 25μm (A-F) and 10μm (G&H). Data represented as mean + SE. Pvalue for superficial and deep plexus is < 0.01.
