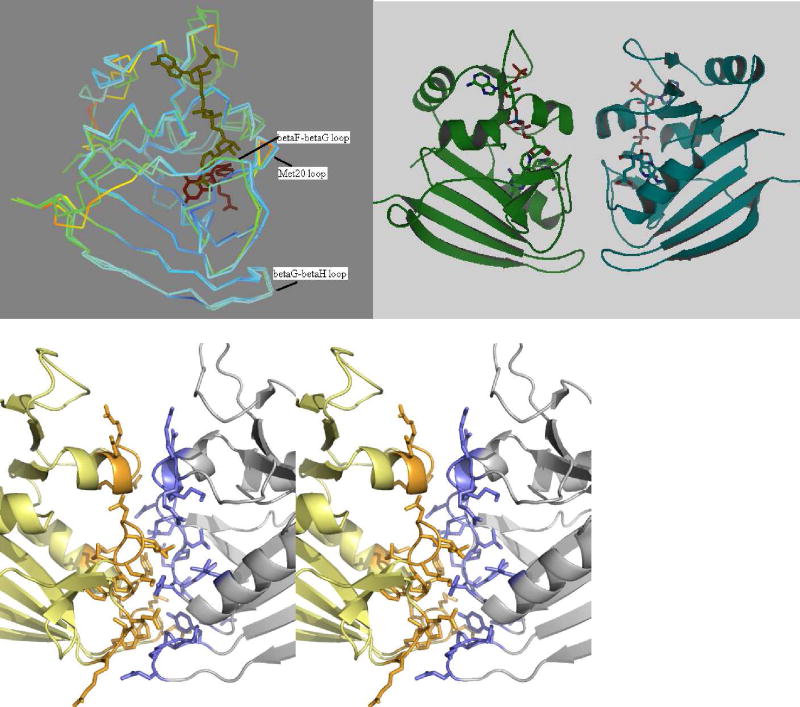
Figure 4.
(a) Superposition of the E. coli and Sp9 DHFR monomers colored according to their temperature factors with red representing mobile regions and blue, the rigid regions. The substrate binding loops and helix in the E. coli structure seem to be more flexible compared to the Sp9 mutant. (b) Cartoon representation of the Sp9 DHFR dimer. (c) Stereo view of the dimer interface. Residues are shown in stick representation.