Figure 2.
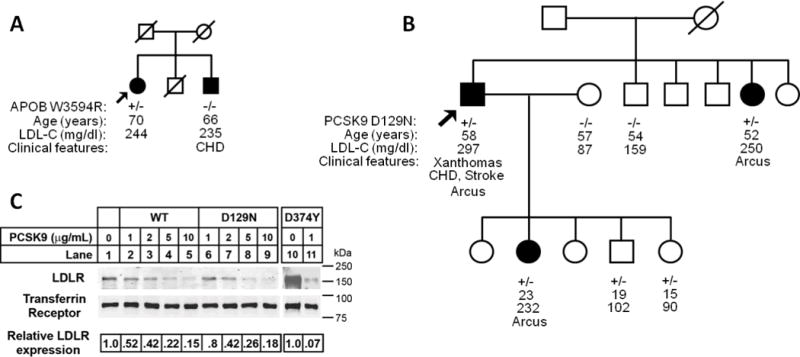
Lack of co-segregation with the hypercholesterolemia phenotype in families found to have APOB and PCSK9 variants. Arrows indicate probands. Filled circle and square designate female and male (respectively) with hypercholesterolemia.A. African American pedigree with APOB p.W3594R heterozygous variant. No disease-causing variants were found in LDLR or PCSK9.B. Hispanic pedigree with PCSK9 p.D129N heterozygous variant. No disease-causing variants were found in LDLR or APOB. C. Degradation of LDLR by exogenous PCSK9 proteins. HepG2 cells were cultured in sterol-supplemented medium (see “Experimental Procedures”) and incubated with the indicated amount of wild-type PCSK9 (WT), PCSK9(D129N) and PCSK9(D374Y) protein for 4 h. Following biotinylation, isolated cell surface protein lysates were subjected to SDS-PAGE and immunoblot analysis. LDLR was detected with an anti-LDLR mAb (HL-1). Transferrin Receptor (TFR) was detected as a control for equal protein loading. Secondary detection used an infrared dye (IRDye800)-labeled antibody. Blots were visualized and quantified using the LI-COR Odyssey infrared imaging system. LDLR levels were normalized to TFR expression. For these data, results shown are from representative experiments repeated at least three times with similar results. CHD denotes coronary heart disease
