Figure 1.
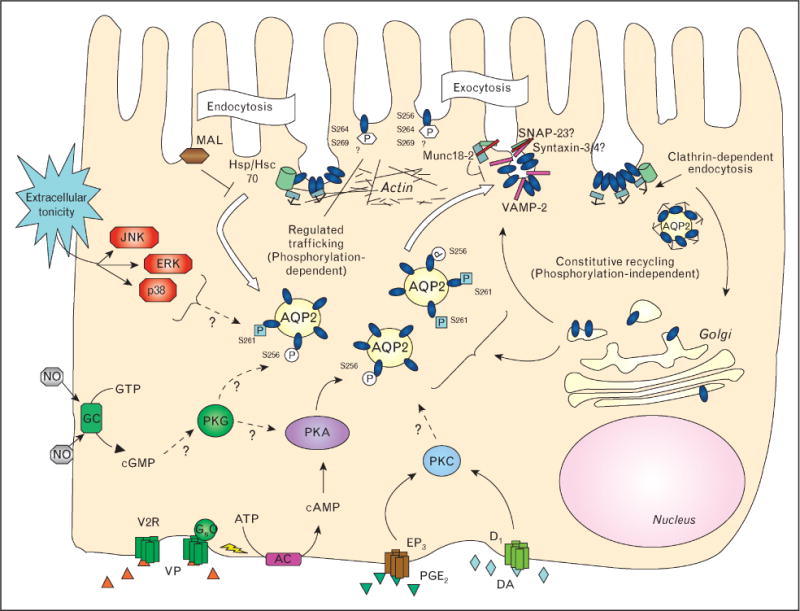
Aquaporin 2 recycling, including endocytosis and both constitutive and regulated exocytosisConstitutive exocytosis occurs independently of aquaporin 2 (AQP2) phosphorylation and involves recycling of AQP2 through a trans-Golgi or recycling endosome compartment. AQP2 membrane accumulation can be increased simply by inhibiting clathrin-mediated endocytosis. The regulated pathway occurs upon vasopressin (VP) interaction with its basolateral receptor (V2R), which increases cAMP formation after Gas stimulation of the adenylyl cyclase (AC). Protein kinase A (PKA) activation results in AQP2 phosphorylation initially on residue S256. At some point, S261 is dephosphorylated and S264 and S269 phosphorylation is increased. AQP2 phosphorylation can also be increased by the cGMP/protein kinase G (PKG) pathways, upon increased activity of the soluble guanylyl cyclase (GC) by, for example, nitric oxide (NO). Extracellular hypertonicity activates the mitogen activated protein (MAP) kinase pathway and JNK, ERK and p38 activities are all required for AQP2 surface accumulation after an acute hypertonic shock. During exocytosis, AQP2 interacts with SNARE proteins and their regulatory proteins such as Munc 18-2, and these interactions may be regulated by phosphorylation. Once at the cell surface, phosphorylated AQP2 resides in endocytosis-resistant domains, and its interaction with hsc70, a protein required for clathrin-mediated endocytosis, is inhibited. AQP2 that is not phosphorylated at S256 interacts strongly with hsc70 and this may be one of several protein–protein interactions that leads to AQP2 accumulation in clathrin-coated pits, followed by endocytosis. The myeloid and lymphocyte-associated protein (MAL) also is involved in AQP2 endocytosis by an as yet unknown mechanism. Endocytosis of AQP2 is also facilitated by protein kinase C (PKC) activation (but possibly not by direct phosphorylation of AQP2), as well as by activation of dopamine (DA, D1) and prostaglandin receptors (EP3, PGE2). Finally, the actin cytoskeleton is centrally involved in AQP2 trafficking: actin depolymerization alone results in cell surface accumulation of AQP2.
