Fig. 2.
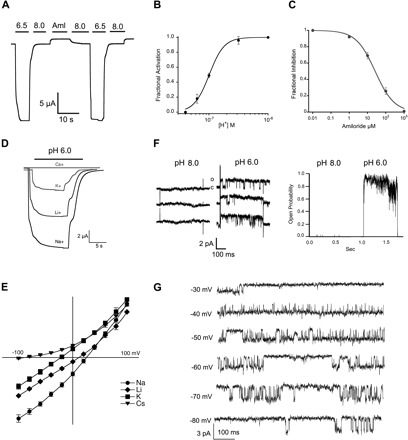
Functional properties of lamprey ASIC1. A: representative current of functional lamprey ASIC1 (3M) evoked by a change in pH from 9.0 to 6.5. A small baseline current was blocked reversibly by 100 μM amiloride (Aml). B: fractional activation by external protons. Whole cell currents of lamprey ASIC1 expressed in Xenopus oocytes were measured by two-electrode voltage clamp (TEVC) in the presence of increasing concentrations of external protons. The lines represent fit of the data to Eq. 1. Each data point is the mean ± SD of 12 cells. C: fractional inhibition by amiloride. Increasing concentrations of amiloride were applied to lamprey ASIC1 currents activated by pH 6.5. The calculated Ki was 26 ± 2 μM, n = 6. D: representative current traces of lamprey ASIC1 activated by pH 6.0 in the presence of 120 mM Na+, Li+, K+, or Cs+ as the only external monovalent cation. Membrane potential, −60 mV. Time (s) and current amplitude (μA) scales are shown with scale bars below traces. E: current-voltage relations of lamprey ASIC1 activated by pH 6.5 in the presence of 120 mM Na+, Li+, K+, or Cs+ as the only external monovalent cation. Data points are normalized currents of at least 4 independent cells. The change in reversal potential was used to calculate the relative permeability to monovalent cations using Eq. 2. F, left: unitary channel currents from an outside-out patch sequentially perfused with pH 8.0 or 6.0. Right: instantaneous open probability calculated from the same patch containing a single channel subjected to 30 consecutive sweeps exposed to pH 8.0 and 46 sweeps to pH 6.0. Membrane potential, −60 mV. G: representative examples of unitary currents evoked by pH 6.8 and recorded at the membrane voltages indicted on the left.
