Figure 4. Expanding the scope of the chaperone interaction assay.
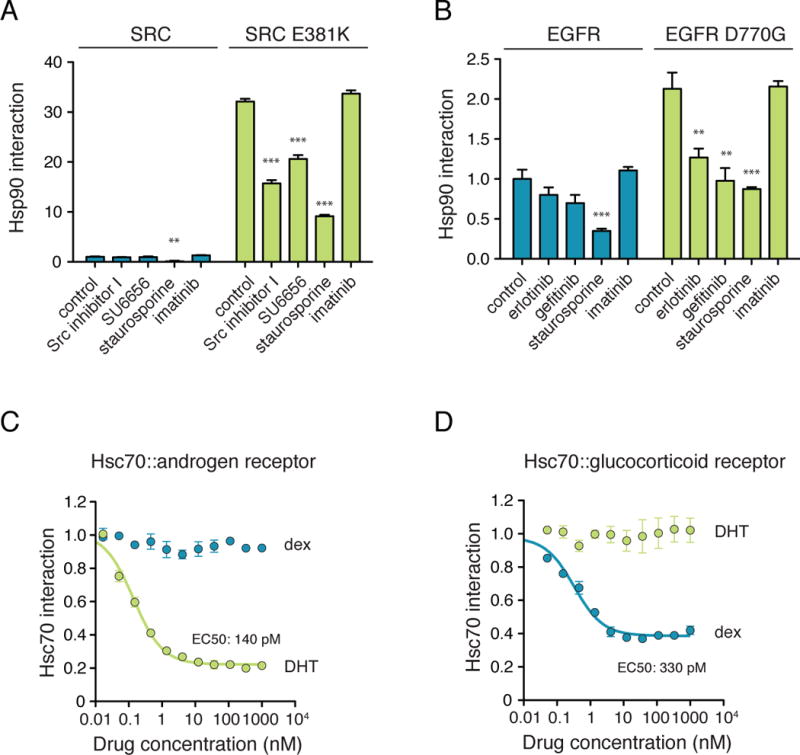
(a) SRC does not appreciably associate with HSP90 in LUMIER assay, and thus, binding of the SRC-specific inhibitors SU6656 and Src inhibitor I to the kinase cannot be measured with LUMIER. Increasing SRC–HSP90 interaction by introducing a point mutation (E381K) to SRC kinase domain enables the detection of SU6656 and Src inhibitor I binding to the kinase. Inhibitors (10 μM) were added to the cells for 1h prior to cell lysis and the LUMIER assay. Interaction scores correspond to relative interactions (Wild type SRC with no inhibitor = 1) +/− SD (n=4). Statistical test for significance was unpaired t-test with unequal variance. **, p<0.01; ***, p<0.001.
(b) Wild-type EGFR associates with HSP90 only weakly. Increasing its interaction with HSP90 with an aspartic acid-to-glycine mutation (D770G) facilitates the detection of binding of the EGFR-specific inhibitors erlotinib and gefitinib to EGFR. Experiment was performed as in (a). **, p<0.01; ***, p<0.001.
(c–d) 293T cells stably expressing Renilla-Hsc70 (HSPA8) were transfected with 3xFLAG tagged androgen receptor (c) or glucocorticoid receptor (d). Cells were treated with increasing concentrations of dexamethasone or dihydrotestosterone (DHT) for 1h prior to cell lysis and LUMIER assay. Relative HSPA8 interaction values (no inhibitor = 1) were fitted to a three-parameter dissociation curve with Graphpad Prism. Error bars indicate standard deviation.
