Abstract
Prestin is the motor protein within the lateral membrane of outer hair cells (OHCs), and it is required for mammalian cochlear amplification. Expression of prestin precedes the onset of hearing in mice, and it has been suggested that prestin undergoes a functional maturation within the membrane coincident with the onset of hearing. We have developed a tetracycline-inducible prestin-expressing cell line that we have used to model prestin's functional maturation. We used prestin's voltage-dependent nonlinear charge movement (or nonlinear capacitance) as a test of function and correlated it to biochemical measures of prestin expressed on the cell surface. An initial stage of slow growth in charge density is accompanied by a rapid increase in our estimate of charge carried by an individual motor. A rapid growth in charge density follows and strongly correlates with an increasing ratio between an apparently larger and smaller monomer, suggesting that the latter exerts a dominant-negative effect on function. Finally, there is a gradual depolarizing shift in the voltage of peak capacitance, similar to that observed in developing OHCs. This inducible system offers many opportunities for detailed studies of prestin.
Keywords: development, prestin, cell lines
mammalian outer hair cells (OHCs) are electromotile, and this property is believed to underlie cochlear amplification, a process that enhances mammalian frequency tuning and sensitivity (4, 5, 16). Prestin, a member of the SLC26 anion transporter family, has been identified as the protein responsible for electromotility (15, 20). In these and other studies, voltage-dependent charge movement of prestin's voltage sensor, detected as a nonlinear capacitance (NLC), has been shown to strongly correlate with electromotility. Thus, NLC can be used as a proxy measure of electromotility. The Boltzmann characteristics of NLC include the density of voltage sensor charge (Qsp), the voltage at peak capacitance (Vh), and unitary charge valence (z). Qsp is an estimate of the number of functional motors within a unit area of surface membrane, Vh is a metric of the steady-state energy profile, and z is an estimate of charge moved within an individual motor. Both electromotility and NLC show a developmental maturation in the OHCs (1, 2, 7, 13). In gerbils, electromotility is first detected at postnatal day P8 and P7 in OHCs from the apical and basal turns, respectively; thereafter it stabilizes at P17–P19 (7), coincident with maturation of hearing (11). In contrast, NLC can be detected as early as P0 in OHCs from the rat apical turn (13). In that study, specific NLC was found to stabilize at P11 well before maturation of hearing in rats (3). However, in mice, linear capacitance (an indicator of both cell surface area and prestin deposition into the membrane) and prestin RNA levels asymptote at about P10, whereas specific NLC continued to increase until P18 (1), which coincides with maturation in hearing (17). The results of Abe et al. (1) strongly suggest a maturation process for the motor protein itself, although the nature of this maturation has yet to be determined. To gain insight into the possible mechanisms underlying this phenomenon, we used a tetracycline-inducible, prestin-expressing stable cell line of human embryonic kidney (HEK) cells to study aspects of NLC as they relate to expression of prestin. Our results demonstrate that maturation following induction involves an increasing incorporation of two monomeric forms of prestin into the cell membrane, with one form exerting a dominant-negative effect on the other. Functional correlates of maturation show early and prolonged time course components following induction, with z increasing during the first few hours, and both Qsp and Vh changing over tens of hours. These data substantiate observations about the maturation of elecromotility in OHCs. They also provide insights that cannot be gleaned from OHC measures alone and underscore the utility of this inducible cell line.
MATERIALS AND METHODS
Prestin gene constructs.
Plasmid pcDNA6/TR is from Invitrogen with selective antibiotic blasticidin. This construct codes for a tetracycline operon (TO) repressor that works with the second plasmid, pcDNA4/TO/myc-HisC, to create a tetracycline-inducible system for protein expression. pcDNA4/TO/myc-HisC is also from Invitrogen and is selective by antibiotic zeocin. The gerbil prestin gene (a gift from J. Zheng and P. Dallos) tagged with enhanced yellow fluorescent protein (EYFP) was inserted into the multiple cloning site of pcDNA4/TO/myc-HisC. This construct (gPrestin-YFP4TOmycHisC) contains a 2× tetracycline operon that works with the first construct to create inducible prestin expression. When tetracycline is absent, the repressor binds to the tetracycline operon, keeping the gene downstream from being expressed. When tetracycline is present, it binds to the repressor, releasing the tetracycline operon and allowing the prestin gene to be expressed. The myc-His tags in the construct are for detection and purification purposes. pEYFP-N1 vector (Clontech) was used to construct the noninducible prestin-YFP-expressing vector with a Flag tag added to the NH2 terminus. This vector uses antibiotic G418 for selecting stable cell lines.
Cell culture and transfection.
HEK293 cells (American Type Culture CollectionATCC, Manassas, VA) were cultured in Dulbecco's modified Eagle's medium (DMEM, high glucose) medium containing 50 U/ml each of penicillin and streptomycin, 10% fetal bovine serum at 37°C in a CO2 incubator (5%). Transfection of constructs into these cells was done using Superfect reagent (Qiagen) according to the manufacturer's instructions for stable transfection. To produce inducible prestin cell lines, pcDNA6/TR was first transfected and selected with 8 μg/ml blasticidin for monoclonal stable cell lines (293-pcDNA6/TR). Then the gPrestin-YFP4TOmycHisC construct was transfected to stabilized 293-pcDNA6/TR cells, and selected with 280 μg/ml zeocin. Stabilized monoclonal prestin-YFP-mycHis expression was initially screened by using fluorescence microscopy 1 or 2 days after addition of 1 μg/ml tetracycline to the growth medium. Culturing of these cell lines (293-TRxST-gPrestin-YFP4TOmycHisC) was in DMEM base medium supplemented with 4 μg/ml blasticidin and 130 μg/ml zeocin. Selection of the Flag-prestin-YFP-N1 construct was done by adding 1,200 μg/ml G418 (geneticin) to these cells. G418 concentration in the growth medium was reduced to 600 μg/ml for stable cell lines.
Patch-clamp electrophysiology.
Intracellular solution (pipette solution) contained (in mM) 136 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES buffer, and 5 EGTA, pH 7.28. Osmolarity was adjusted to 299 ± 2 mosM using glucose for all solutions. Extracellular solution (bath solution) contained (in mM) 20 TEA, 20 CsCl, 2 CoCl2, 1.47 MgCl2, 10 HEPES buffer, 99.2 NaCl, 2 CaCl2, and 2 BaCl2, pH 7.28. Glass pipettes were pulled using a P-2000 laser-heating pipette puller (Sutter Instruments). All pipettes have initial resistances of about 2.5 MΩ. All patches were made on single cells growing on a coverslip at different time points after tetracycline induction. A Nikon Eclipse FNI upright microscope equipped with a ×40 water immersion lens and a green fluorescent protein (GFP) UV light filter was used for cell observation, and an EXFO motorized manipulator by Burleigh (PCS-6000) was used for pipette manipulation. Data acquisition was done with a Digidata 1322A A/D converter and an Axonpatch 200B Integrating Patch clamp (Axon Instruments). Cell capacitance was measured under whole cell configuration using the jClamp software (http://www.scisoftco.com/). For time course studies with tetracycline-inducible cell lines, 4–11 cells were averaged for each time point. NLC from two inducible cell lines was measured separately, and the results are comparable. Only one is presented in this study.
The NLC traces show the change of membrane capacitance Cm in response to voltage across the membrane Vm. Each trace can be fitted according to Eq. 1 using four parameters:
where
| (1) |
Qmax is maximum nonlinear charge transfer, Vh is voltage at peak capacitance or half-maximal nonlinear charge transfer, Vm is membrane potential, Clin is linear capacitance proportional to cell surface area, z is valence (a metric of voltage sensitivity), e is electron charge, k is Boltzmann's constant, and T is absolute temperature. Qmax is reported as Qsp, the specific charge density, i.e., total charge moved normalized to linear capacitance. Similarly, specific nonlinear capacitance NLCsp refers to (Cm − Clin)/Clin.
Confocal fluorescence imaging.
Tetracycline (1 μg/ml) was added to the cell growth medium 1 day after the cells were plated on coverslips. After incubation for 24 to 48 h for tetracycline-induced expression of prestin-YFP, cells were washed briefly with PBS, then fixed in 3% formaldehyde in PBS for 20 min at room temperature. Following a wash in PBS, cells were mounted with VectaShield mounting media for confocal microscope observation. All confocal experiments were done on a Zeiss LSM 510 Meta confocal microscope using a 514 nm laser line. Image analysis was done using LSM Image browser.
To analyze fluorescence intensity of cell surface prestin versus total prestin expression using YFP fluorescence imaging, individual cells from the 293-Flag-prestin-YFP cell line were analyzed using Photoshop's histogram utility. The integrated fluorescence intensity is the product of mean pixel intensity, after subtracting mean background intensity, times total pixel numbers in the region of interest (ROI). By demarcating the outer edge of the whole cell as the ROI, we obtained total integrated pixel intensity. We then obtained intracellular intensity by applying the same algorithm defining the ROI within the inner aspect of the cell membrane. Surface intensity was obtained by subtracting the intracellular intensity from the total cell intensity.
Electron microscopy of nanogold-labeled prestin-YFP.
Electron microscopy immunohistochemistry was done at the Yale Center for Cell and Molecular Imaging using a Tecnai 12 Bio Twin Transmission Electron Microscope. All images were taken at the same calibrated magnification (×43,000). Fifty images were taken randomly from HEK cells expressing prestin-YFP, as well as 20 images from a control sample (untransfected HEK cells) for quantification and statistics. For all images included in this analysis, the anti-GFP primary antibody conjugated with 10-nm nanogold was used at a 1:50 dilution for maximum labeling efficiency. Background labeling as seen in mitochondria and nucleus was very low. Quantification of surface prestin and total prestin expression was done by counting the nanogold particles either on plasmic membrane only or everywhere in the cell.
Surface protein labeling.
Cell surface protein labeling was done according to the manufacturer's instructions (Pierce Biotech, EZ-link Sulfo-NHS-SS-Biotin surface protein isolation kit). Briefly, cells grew on 10-cm culture plates, and 1 μg/ml tetracycline was added at different time points to give different incubation times at harvest. Cell surface proteins were labeled after a brief wash in cold PBS at 4°C on a flat shaker. The reaction was quenched using quenching solution after 30 min, and cells harvested by using a scraper. Cells were spun down and briefly washed in Tris-buffered saline before addition of lysis buffer supplemented with protease inhibitors (Roche Complete protease inhibitors). Cells were lysed at 4°C for 30 min with end-to-end rotation, and total protein was quantified using Bio-Rad Protein Assay kit (500–0006); 2,500 μg of total protein from each sample was mixed with 250 μl NeutrAvidin Gel and incubated for 1 h at room temperature. Following steps of washing and centrifugation, plasma membrane proteins were purified and eluted from the gel using SDS sample buffer with 100 mM DTT. Two microliters of each eluted surface protein was loaded onto a Bio-Rad 4–15% Tris-glycine gradient gel. Lysate loading was also quantified to have 0.75 μg of total protein for each lane. In total, three rounds of surface labeling were done, each followed by Western blot and densitometry measurements, and the results were averaged. To avoid signal saturation on the film, and to minimize the subjectivity in assigning the boundaries between the bands, different exposures that gave clearly identifiable and separate bands were used. To allow comparison between different exposures, data were normalized to several bands in the middle lanes with expression levels that were intermediate.
RESULTS
We have established several inducible and noninducible HEK cell lines stably expressing the motor protein prestin. When making these cell lines, we used a number of prestin constructs where prestin was variably tagged to YFP, and the c-myc and FLAG epitopes. We have studied the distribution of prestin expression in these cells by electron microscopic immunogold labeling and confocal imaging. Using our tetracycline-inducible cell line, we investigated the development of the motor protein by measuring NLC and correlated these data with biochemical measures of prestin surface expression in these cells.
Prestin is targeted to the surface membrane in HEK cells.
With both inducible and noninducible cell lines expressing prestin-YFP, we observed that YFP fluorescence was localized to the plasma membrane, but significant amounts remained intracellularly (Fig. 1). In inducible cell lines, YFP was observed at very low levels before induction (Fig. 1F) but showed a dramatic increase after induction (Fig. 1, D and E).
Fig. 1.
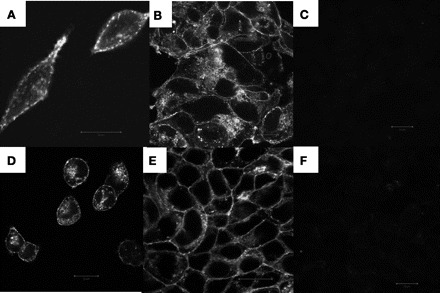
Prestin-yellow fluorescent protein (YFP) is localized to the membrane. Prestin-YFP expression in human embryonic kidney (HEK) 293 cell lines characterized by confocal imaging of YFP fluorescence is shown. A and B: a HEK293 cell line stably expressing a Flag-tagged prestin-YFP construct. C: untransfected HEK293 cells as negative control. D–F: a tetracycline-inducible HEK293 cell line expressing prestin-YFP with a mycHis6 tag at its COOH terminus. D and E: 24 h after addition of 1 μg/ml tetracycline to the growth media. F: no tetracycline added as negative control. Scale bar, 20 μm.
We also attempted to better localize prestin in these cell lines by using nanogold labeling with an antibody to GFP that also recognizes YFP with high fidelity (Fig. 2). In these experiments, we noted that nanogold particles were localized on the plasma membrane and within the cell. A quantitative morphometric analysis where individual particles were counted in 50 photomicrographs revealed that about half of the particles were on the surface membrane of the cell (Fig. 3A). These data are congruent with measurement of fluorescence in YFP-tagged prestin-expressing cells. In these cells, ∼50% of the fluorescence signal was localized to the surface of the cell (Fig. 3B).
Fig. 2.
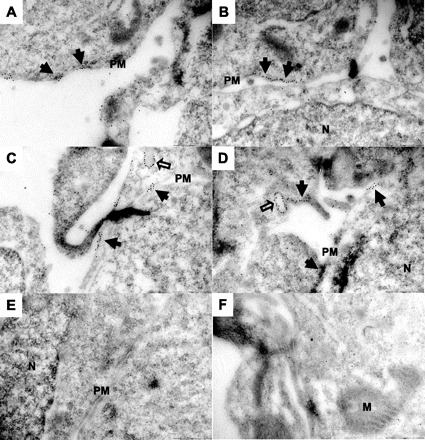
Immunogold labeling localizes prestin to the plasma membrane. Electron microscopy (EM) images of a HEK293 cell line expressing Flag-tagged prestin-YFP are shown. Cells were grown to a monolayer in culture dishes, fixed, embedded, thin-sectioned, labeled with anti-green fluorescent protein (GFP) antibody (that cross-reacts with YFP) conjugated with 10 nm nanogold particles, stained, and imaged using a Tecnai 12 Bio Twin transmission electron microscope. A–D: samples from cells expressing prestin-YFP. Nanogold labeling is clearly seen on the plasma membrane (filled arrows), indicating proper trafficking and targeting of prestin molecules to the cell membrane. Notice also scattered labeling in the cytoplasm and on some intracellular vesicles as pointed out by hollow arrows. E and F: in the untransfected HEK293 cells, very small amount of labeling was observed. N, nucleus; PM, plasma membrane; M, mitochondria. Scale bar, 250 nm.
Fig. 3.
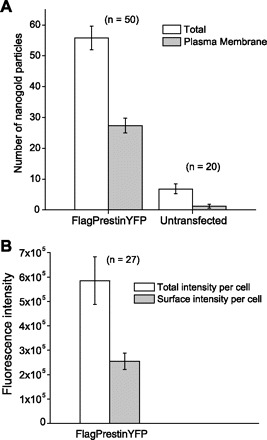
Equivalent amounts of prestin are found in the plasma membrane and cytoplasm. Quantitative analysis on the distribution of prestin molecules in a Flag-tagged prestin-YFP stable cell line is shown. A: nanogold particle counting from EM images. Individual particles were counted from each micrograph, and the particles on the plasma membrane were subtracted from the total. As shown here, on average there are 56 gold particles in each micrograph of transfected cells, out of which 27 are localized on the cell membrane (∼50.6 ± 2.5%). B: the intensity of fluorescence was assayed on Flag-tagged prestin-YFP-expressing cells. The localization of YFP fluorescence was quantified in the plasma membranes of these cells. The plasma membranes of these cells contained 48.6 ± 1.9% of the total fluorescence signal (± SE).
Nonlinear capacitance characterization of stable cell lines.
Fluorescence measurements indicate that our stable cell lines have high levels of prestin-YFP located in the cell plasma membrane. We then tested these cell lines for functional expression of prestin, by determining NLC in several representative cells from each cell line.
Under whole cell voltage-clamp configuration, we were able to record large NLC in most of our HEK cell lines expressing prestin (Fig. 4). For example, a Flag-tagged noninducible cell line (termed G) exhibits a peak NLC of 3.7 pF (Fig. 4, top trace), while the tetracycline-inducible line (termed 16c) has a peak NLC of 2.8 pF (bottom trace). NLC values of these cell lines are summarized in Table 1. These typical values are higher than values seen in transiently transfected Chinese hamster ovary cells, which exhibit peak capacitance of about 1 pF; expression levels in these lines are also higher than other stable prestin cell lines (9).
Fig. 4.

Large nonlinear capacitance (NLC) is generated in cell lines expressing prestin. Shown are typical traces of NLC from two stable cell lines expressing prestin-YFP. The data were fitted according to Eq. 1. Top: trace from a noninducible HEK293 cell line (termed G) expressing Flag-tagged prestin-YFP. The fitting parameters were as follows: peak capacitance voltage (Vh) = −66.1 mV, unitary charge valence (z) = 0.801, maximum nonlinear charge (Qmax) = 0.468 pC, and linear capacitance (Clin) = 9.61 pF. Cm, membrane capacitance. Bottom: NLC trace from a tetracycline (Tet)-inducible HEK293 cell line expressing prestin-YFP tagged with mycHis6. Tetracycline (1 μg/ml) was added to the growth media, and NLC was measured 34 h after tetracycline induction. Fitting parameters were as follows: Vh = −75.3 mV, z = 0.804, Qmax = 0.365 pC, and Clin = 10.3 pF. Vm, cell membrane potential.
Table 1.
Nonlinear capacitance parameters from representative prestin cell lines
| Cell Line | Qsp, fC/pF | Vh , mV | z | n | Notes |
|---|---|---|---|---|---|
| Flag-prestin-YFP-G* | 20.1 ± 2.7 | −106 ± 7.0 | 0.78 ± 0.04 | 5 | |
| Prestin-YFP-myc-His-15b† | 14.1 ± 1.4 | −93.9 ± 5.2 | 0.70 ± 0.02 | 7 | 30 h Tet |
| Prestin-YFP-myc-His-16c† | 20.6 ± 1.3 | −77.0 ± 7.9 | 0.76 ± 0.01 | 8 | 28 h Tet |
| HEK transiently transfected cells (18) | ∼11 ± 4 | −70 ± 18 | 0.82 | 10 | |
| CHO cell line (8) | ∼6 | −75 |
Values are means ± SE of nonlinear capacitance parameters in one noninducible
and two tetracycline (Tet)-inducible
monoclonal cell lines we have developed. For inducible cell lines 15b and 16c, values were chosen from specific time points after induction, since their specific charge density (Qsp) as well as unitary charge valence (z) and voltage at peak capacitance (Vh) change with time of incubation. These cells have a range of Qsp values, all of which are higher than either typical transiently transfected human embryonic kidney (HEK) cells or other stable cell lines developed previously. YFP, yellow fluorescent protein; CHO, Chinese hamster ovary cells. Reference numbers are in parentheses.
Development of NLC in a tetracycline-inducible prestin cell line.
We used tetracycline-inducible cell lines to analyze time-dependent changes in NLC. Typical traces of NLC from different time points after addition of tetracycline to the growth media are shown in Fig. 5. When no tetracycline is added, little or no NLC is observed. With 1 μg/ml tetracycline, NLC becomes readily measurable as early as 2 h after addition.
Fig. 5.
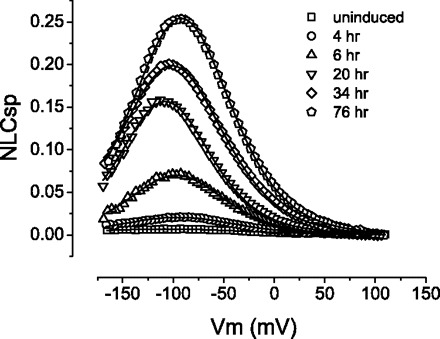
NLC increases as a function of time in inducible cell lines expressing prestin. Shown are representative NLC curves from a tetracycline-inducible HEK293 cell line (16c). Specific NLC (NLCsp) was used as a means to normalize data to cell size [NLCsp = (Cm − Clin)/Clin]. Without tetracycline added to the growth media (uninduced), prestin expression is repressed, resulting in minimal measurement of NLC. As the incubation time with tetracycline lengthens, the cells develop higher NLC as a result of either more prestin expression and/or prestin maturation on the cell membrane. Fitting parameters according to Eq. 1 were the following: for 4 h, Vh (mV) = −93.8, z = 0.697, Qmax (pC) = 0.045, Clin (pF) = 18.0; for 6 h, Vh = −97.3, z = 0.799, Qmax = 0.213, Clin = 23.7; for 20 h, Vh = −108, z = 0.855, Qmax = 0.270, Clin = 14.6; for 34 h, Vh = −101, z = 0.753, Qmax = 0.528, Clin = 19.5; and for 76 h, Vh = −92.2, z = 0.780, Qmax = 0.548, Clin = 16.5.
We also assayed three different parameters of NLC, Qsp, z, and Vh, to further delineate prestin function. Qsp, charge movement per unit of membrane surface, increases with time in a sigmoidal manner (Fig. 6A). The inset in Fig. 6A shows the growth phase in the first 6 h after addition of tetracycline. This phase was characterized by a steady, yet slow increase of Qsp, to about one-tenth of the asymptotic value. This is followed by a rapid increase in Qsp from 6 h to about 20 h after tetracycline induction, where Qsp increases to ∼60% of the asymptotic value. Following this rapid increase there is again a phase of slow increase in Qsp to its mature level over a further period of 72–96 h.
Fig. 6.

NLC parameters change as a function of time after induction. A: development of specific charge density (Qsp) after induction in a representative tetracycline-inducible prestin cell line (16c) with time. Qsp increase shows a sigmoidal pattern with time and stabilizes at 30+ h. The number of cells patched for each point ranged from 4 to 11. Error bars are ±SE. Data were fitted to a Hill equation [y = start + (end − start) × x∧n/(k∧n + x∧n)] with the following parameters: start = 1.23; end = 32.0; k = 18.3; n = 2.0. B: development of unitary charge valence (z) in a tetracycline-inducible cell line as a function of time after induction. z shows a rapid increase over several hours to reach its plateau at 6–8 h. z values did not change significantly, remaining stable after this time point. Fitting parameters were as follows: start = 0.58; end = 0.80; k = 3.0; n = 6.8. C: development of voltage dependence of peak NLC (Vh) as a function of time after induction. While there was considerable variation in Vh values, there was a clear trend in the development of Vh values toward more depolarizing voltages with time. Fitting parameters were as follows: start = −123; end = −69.4; k = 8.6; n = 0.74.
In contrast to Qsp, which takes over 60 h to reach its asymptotic level, z, an estimate of charge carried by an individual motor, reaches a maximum level of 0.8e 6 h after induction (Fig. 6B).
We also looked at changes in Vh over the time course of prestin expression. As shown in Fig. 6C, even though Vh values fluctuate, there was a general trend for these values to reach more depolarized potentials with time. Vh settles near −80 mV at ∼30 h after tetracycline induction.
Development of surface prestin expression in a tetracycline-inducible cell line.
We undertook quantitative surface protein labeling experiments using a tetracycline-inducible cell line to determine the expression of prestin on the cell surface. We used a cell surface protein labeling method with cleavable water-soluble Sulfo-NHS-SS-Biotin reagent that then allows for the isolation of surface-labeled protein through its binding to avidin-Sepharose beads. The amount of prestin in these surface-labeled preparations was then determined using Western blotting.
Prestin expressed on the cell surface reaches an asymptotic level at 18–20 h (Fig. 7, B and D). In contrast, the total amount of prestin in the cell continues to increase throughout the sampling period after induction, albeit more slowly after 22 h (Fig. 7, A and C). In these experiments, we observed two major monomeric forms of prestin in both total cell lysate and the cell surface components. The upper monomer has a molecular mass of ∼125 kDa, and the lower monomer a mass of ∼100 kDa. There is also a broad multimeric form of prestin, which was first detected at ∼4 h after induction. The upper monomeric form is the predominant component in both cell lysates and plasma membranes. When plotting Qsp against the intensity of the upper monomer alone, we obtained a linear correlation (adjusted R2 = 0.83). However, this relationship was attenuated at higher Qsp values, when the amount of upper monomer remained stable with continued increase in Qsp (Figs. 7D and 8A). At higher Qsp values, there was a decrease in the lower monomer (Fig. 7D). Concordant with these data, we observed a strong linear relationship (with adjusted R2 = 0.92, Fig. 8A), when we plotted Qsp against the ratio of the upper to lower monomer. These results corroborate our previous assertion (9) that the lower monomer has a dominant-negative effect on the upper monomer. Interestingly, there is also a linear correlation between the initial rapid rise in z and the shift toward more depolarizing voltages in Vh (Fig. 8B). We note that this particular correlation was not an artifact of different initial fitting parameters. That is, we tested a range of voltages used to bracket NLC and established that Vh was not changed by the limits of the voltage range set when establishing a fit.
Fig. 7.
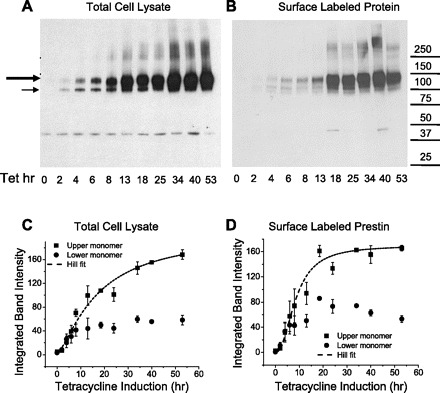
Two monomers and multimers of prestin develop differentially as a function of time after induction (cell line 16c). A: Western blot of total cell lysate probed with anti-prestin N-20 antibody. Each lane has 0.75 μg of total protein as determined by protein assay. The large and small arrows indicate the upper (dominant) and lower monomers of prestin-YFP, respectively. A dimeric form of the protein is identifiable at 250 kDa. Prolonged incubation with tetracycline causes total cell prestin expression to increase steadily. The band at ∼40 kDa is a nonspecific artifact, because it appears also in untransfected HEK293 cells (data not shown). B: Western blot of surface-labeled prestin-YFP obtained from cells at different time points after induction with 1 μg/ml tetracycline. Each lane has 2 μl of the 250 μl surface-purified eluate from NeutrAvidin beads after binding to 2.7 mg of total protein. Each lane was derived from and corresponds to cell lysates in A. As with total cell lysates, prestin has at least two monomers and a broad dimeric form. The monomers in particular are detectable at the earliest time point (2 h) at which NLC is detectable. C and D: densitometric quantification of monomers of prestin from Western blots reveals a sigmoidal increase in the upper larger monomer of prestin. The surface expression of this monomer, however, stabilizes earlier than the monomer in cell lysates, with an asymptotic value achieved at 20 h after induction. The lower monomer expressed on the surface in contrast shows a small but persistent decrease in the asymptotic value with time. Hill fitting parameters for lysate (C) were as follows: start = 1; end = 205; k = 17.2; n = 1.4. Hill fitting parameters for surface prestin (D) were as follows: start = 0; end = 169; k = 8.6; n = 2.2. Data are averages from three batches of surface labeling experiments. Error bars are ±SE.
Fig. 8.
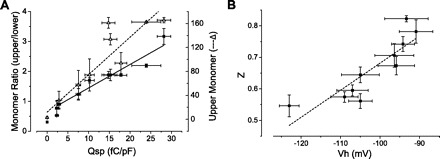
Prestin's lower monomer exerts a dominant-negative effect on the upper functional monomer. A: the relationship between Qsp and the densitometric ratio of the upper to lower prestin monomer expressed on the surface of the cell is linear (Qsp = −8.35 +11.12 × ratio; adjusted R2 = 0.93). Data were obtained from cells at variable time points after induction. Taken together, these data would suggest that the lower monomer exerts a dominant-negative effect on the function of the molecule. Also shown is the relationship between the upper monomer intensity and Qsp. There is an initial correlation between the two that decreases with higher Qsp values. B: correlation of z and Vh in the initial phase after induction where there is a rapid increase in z that correlates with a change in Vh.
DISCUSSION
We have developed and used an inducible HEK cell line as a model system to understand prestin's maturation that has been observed in OHCs (1, 13). We used a combination of imaging, electrophysiological recording, and protein labeling to ascertain aspects of prestin's development. Both confocal fluorescence and electron microscopy techniques show prestin to be efficiently targeted to the surface of the cell, with approximately half of the expressed prestin found on the cell surface. Immunogold labeling and fluorescence quantifications were equivalent.
We also observed a relationship between aspects of prestin biochemistry and function. Increase in Qsp, which corresponds to electromotility, clearly correlates to an increase in the level of prestin expression on cell surface (Fig. 8A). Moreover, consistent with our prior data in transiently transfected cells (9), we observed a strong linear relationship between Qsp and the ratio of the upper and lower forms of prestin monomer. These data indicate that the lower prestin monomer has a dominant-negative effect on the upper monomer. It also means that the lower monomer is a less functional or nonfunctional variant of prestin. Moreover, because they imply interactions between upper and lower forms, these data are indirect evidence that the functional unit of the protein is a dimer, in line with previous suggestions (6, 12, 14, 19).
Our data raise questions as to the nature of the two monomers of prestin that we identified. Three scenarios are conceivable. First, it may be that the two monomers are differentially folded forms of the protein, with the smaller being misfolded and migrating faster on SDS-PAGE. This possibility is supported by our previous data showing increasing amounts of the lower monomer with cysteine mutants that are likely important for the structural integrity of the protein (9), The second scenario, that the lower monomer represents proteolytic cleavage of the protein, is untenable since antibodies to both NH2- and COOH-terminal tags of the protein identify both monomers (data not shown). Lastly, it is also possible that these two monomers represent effects of differential glycosylation (10) with the upper and lower monomer representing glycosylated and unglycosylated forms of the protein, respectively. However, since we and others have previously shown that wild-type prestin and prestin in which the two potential N-glycosylation sites (N163 and N166) were mutated differ minimally in function (10, 12), this possibility will not explain the dominant-negative effect of the lower monomer on prestin function (Fig. 8A).
Our hypothesis that the lower monomer represents a misfolded form raises mechanistic questions concerning prestin turnover. Induction results in a continuous production of prestin that continues in time beyond asymptotic measures of prestin activity (Qsp), indicating a mechanism of prestin removal. Since the ratio of upper to lower monomer changes with time until steady state, we reason that the rates of production and/or removal of these two forms of the monomer must be different during the early and late stages following induction. This possibility is substantiated by the differing rates of change in upper and lower monomers that occur over time in total cell lysates versus that in the plasma membrane (Fig. 7, C and D).
We noted an early increase in z after induction (Fig. 6B), indicating that the unitary charge carried by a functional motor changes with time. Since there are data supporting the role of the dimer as the functional unit (6, 12, 14, 19), an obvious possibility is that the increase in z represents a rapid increase of dimers on the cell surface in the initial hours of induction. We were unable to substantiate this possibility owing to technical difficulties in detecting prestin dimer in the very early stages after induction (0–4 h), when z shows its greatest change.
Our detection of a progressive depolarizing shift in Vh is similar to that observed in OHCs (1, 13). It had been suggested that this shift might represent development of other unique molecular structures within the OHC, such as subsurface cisternae, pillars, and cortical cytoskeleton (1). Since a similar developmental event is unlikely in HEK cells, a more plausible explanation is that this change in Vh represents a maturation process that is intrinsic to the protein. It is interesting that in the first 6 h after induction, this shift in Vh coincides with the rapid increase in z (Fig. 8B). This suggests that charge valence and sensitivity to voltage is coupled in the initial stages of prestin's maturation.
In conclusion, we have developed several stable cell lines that have functional expression of prestin up to four times that of transiently transfected cells and have focused on a tetracycline-inducible line to elucidate the molecular events that underlie the development of prestin's NLC characteristics. Our data suggest that two prestin monomers are expressed on the cell surface, only one form being functional. Our data also suggest that dimerization of prestin may be associated with increases in prestin's voltage sensitivity (z values). Finally, our data suggest that prestin undergoes an intrinsic maturation that results in a shift in its voltage operating range to more depolarizing levels. Clearly, these cell lines will be useful as model systems for studying other aspects of prestin activity, including trafficking and turnover.
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grants DC-007894, DC-00273, and DC-008130.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Abe T, Kakehata S, Kitani R, Maruya S, Navaratnam D, Santos-Sacchi J, Shinkawa H. Developmental expression of the outer hair cell motor prestin in the mouse. J Membr Biol 215: 49–56, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belyantseva IA, Adler HJ, Curi R, Frolenkov GI, Kachar B. Expression and localization of prestin and the sugar transporter GLUT-5 during development of electromotility in cochlear outer hair cells. J Neurosci 20: RC116, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blatchley BJ, Cooper WA, Coleman JR. Development of auditory brainstem response to tone pip stimuli in the rat. Brain Res 429: 75–84, 1987 [DOI] [PubMed] [Google Scholar]
- 4.Brownell WE, Bader CR, Bertrand D, de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science 227: 194–196, 1985 [DOI] [PubMed] [Google Scholar]
- 5.Dallos P, Wu X, Cheatham MA, Gao J, Zheng J, Anderson CT, Jia S, Wang X, Cheng WH, Sengupta S, He DZ, Zuo J. Prestin-based outer hair cell motility is necessary for mammalian cochlear amplification. Neuron 58: 333–339, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Detro-Dassen S, Schanzler M, Lauks H, Martin I, zu Berstenhorst SM, Nothmann D, Torres-Salazar D, Hidalgo P, Schmalzing G, Fahlke C. Conserved dimeric subunit stoichiometry of SLC26 multifunctional anion exchangers. J Biol Chem 283: 4177–4188, 2008 [DOI] [PubMed] [Google Scholar]
- 7.He DZ, Evans BN, Dallos P. First appearance and development of electromotility in neonatal gerbil outer hair cells. Hear Res 78: 77–90, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Iida K, Tsumoto K, Ikeda K, Kumagai I, Kobayashi T, Wada H. Construction of an expression system for the motor protein prestin in Chinese hamster ovary cells. Hear Res 205: 262–270, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Bai JP, Surguchev A, Bian S, Song L, Santos-Sacchi J, Navaratnam D. Combinatorial cysteine mutagenesis reveals a critical intra-monomer role for cysteines in prestin's voltage sensing. Biophys J. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuda K, Zheng J, Du GG, Klocker N, Madison LD, Dallos P. N-linked glycosylation sites of the motor protein prestin: effects on membrane targeting and electrophysiological function. J Neurochem 89: 928–938, 2004 [DOI] [PubMed] [Google Scholar]
- 11.McFadden SL, Walsh EJ, McGee J. Onset and development of auditory brainstem responses in the Mongolian gerbil (Meriones unguiculatus). Hear Res 100: 68–79, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Navaratnam D, Bai JP, Samaranayake H, Santos-Sacchi J. N-terminal-mediated homomultimerization of prestin, the outer hair cell motor protein. Biophys J 89: 3345–3352, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliver D, Fakler B. Expression density and functional characteristics of the outer hair cell motor protein are regulated during postnatal development in rat. J Physiol 519: 791–800, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasqualetto E, Seydel A, Pellini A, Battistutta R. Expression, purification and characterisation of the C-terminal STAS domain of the SLC26 anion transporter prestin. Protein Expr Purif 58: 249–256, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Santos-Sacchi J, Shen W, Zheng J, Dallos P. Effects of membrane potential and tension on prestin, the outer hair cell lateral membrane motor protein. J Physiol 531: 661–666, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santos-Sacchi J, Song L, Zheng J, Nuttall AL. Control of mammalian cochlear amplification by chloride anions. J Neurosci 26: 3992–3998, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song L, McGee J, Walsh EJ. Development of cochlear amplification, frequency tuning, and two-tone suppression in the mouse. J Neurophysiol 99: 344–355, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Sturm AK, Rajagopalan L, Yoo D, Brownell WE, Pereira FA. Functional expression and microdomain localization of prestin in cultured cells. Otolaryngol Head Neck Surg 136: 434–439, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng J, Du GG, Anderson CT, Keller JP, Orem A, Dallos P, Cheatham M. Analysis of the oligomeric structure of the motor protein prestin. J Biol Chem 281: 19916–19924, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature 405: 149–155, 2000 [DOI] [PubMed] [Google Scholar]


