Fig. 2.
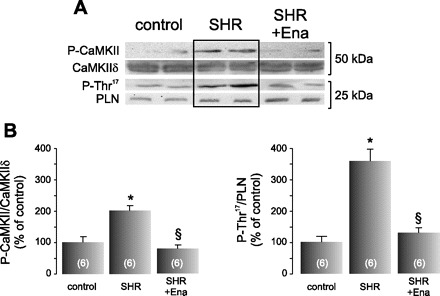
Ena blunts Ca2+-calmodulin-dependent protein kinase II (CaMKII) activity in SHR. A: typical blots of the phosphorylated form of CaMKII (P-CaMKII) and of its substrate, phospho-Thr17 residue of phospholamban (PLN; P-Thr17) in control rats, SHR, and SHR treated with Ena. Total CaMKIIδ and PLN expression are also shown. SHR depicted an increment in both phosphor-proteins, and the treatment with Ena prevented both phosphorylations. B: average data of these experiments. The signals of P-CaMKII and P-Thr17 were normalized by their total respective proteins. These experiments revealed that CaMKII is being activated by ANG II. Values are means ± SE; n, no. of animals in each experimental group (in parentheses). *P < 0.05 vs. control. §P < 0.05, +Ena vs. SHR.
