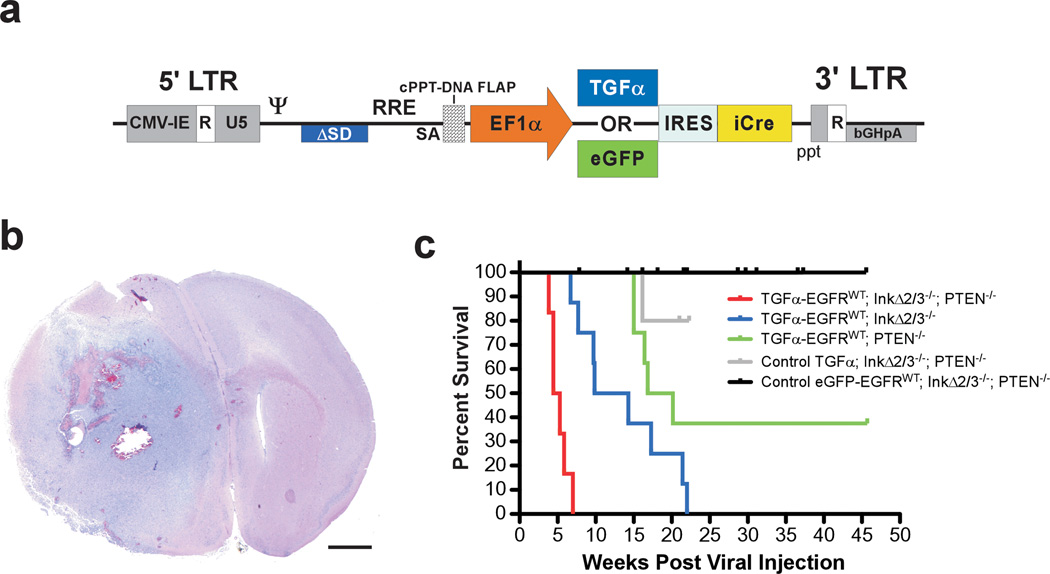
Figure 1.
EGFRWT cooperates with loss of tumor suppressor genes to form brain tumors. (a) Schematic representation of the self-inactivating bicistronic lentiviral transducing vectors. These vectors carry a cassette composed of either the human TGFα cDNA or enhanced green fluorescent protein (eGFP) gene (for control experiments) followed by a human poliovirus1 internal ribosomal entry site (IRES) and improved Cre recombinase (iCre) cDNA (Shimshek et al., 2002) driven by the human elongation factor-1α (EF1α) promoter. The message is stabilized by the presence of the bovine growth hormone poly adenylation signal sequence. These lentiviral vectors were derived from a previously described vector (Coleman et al., 2003). The presence of a central polypurine tract (cPPT)-DNA FLAP element upstream of the multiple cloning site significantly improves the transduction efficiency in CNS tissues (Follenzi et al., 2000; Zennou et al., 2001). LTR; long terminal repeat. (b) Photomicrograph of an H&E-stained coronal section of a representative TGFα-EGFRWT;InkΔ2/3−/−;PTENlox brain tumor. Scale bar; 1.0 mm. (c) Survival (Kaplan-Meier) analysis of conditional EGFRWT mice. Cohorts of mice of the indicated genotypes were stereotactically injected in the striatum with titer-matched pTyf-TGFα-IRES-iCre or pTyf-eGFP-IRES-iCre viruses and monitored for survival over time.