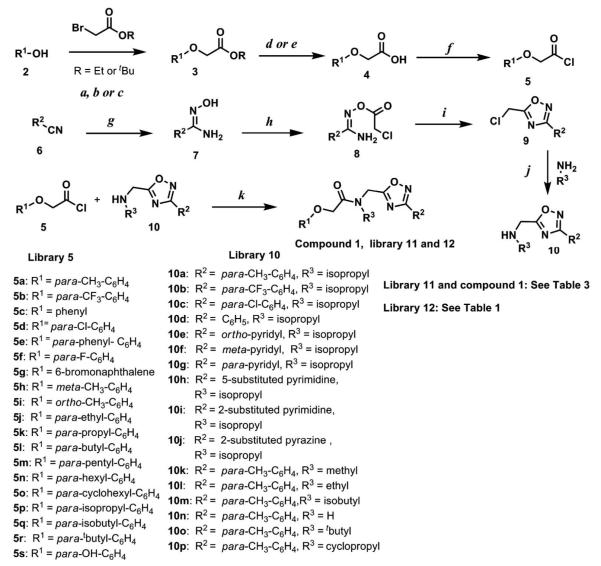
Scheme 1.
Synthetic route to compound 1, libraries 11 and 12. Reagents and conditions: a. Ethyl bromoacetate, K2CO3, Acetone, reflux, 14 h. b. tert-Butyl bromoacetate, DMF, 80 °C, 14 h. c. Ethyl bromoacetate, K2CO3, DMF, r.t., 14 h. d. NaOH, THF, reflux, 2 h. (R= Ethyl). e. CF3COOH, DCM, r.t., 2 h (R = tBu). f. SOCl2, benzene, reflux, 3 h. g. NH2OH.HCl, Na2CO3, water, 70 °C, 14 h. h. Chloroacetyl chloride, acetone, r.t., 30 min. i. toluene, reflux, 2 h. j. Alkylamine, K2CO3, CH3CN, reflux, 30 min. k. Et3N, THF, r.t., 15 min.