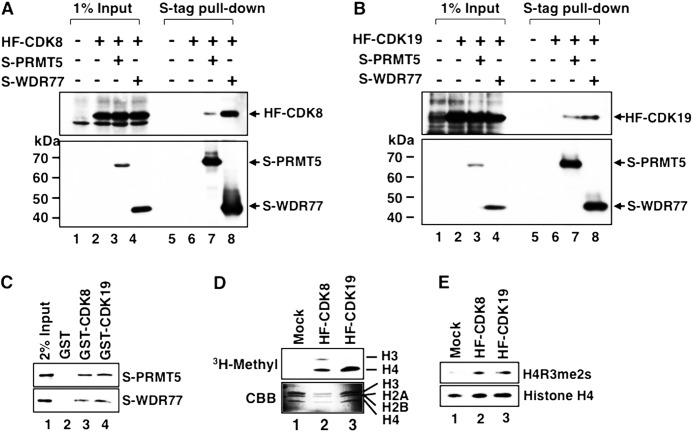
FIGURE 4.
Binding of PRMT5 and WDR77 to Mediator CDKs and detection of histone H4 arginine 3 symmetric dimethylation (H4R3me2s) activity in CDK-containing Mediator complexes. A and B, S-tagged PRMT5 (S-PRMT5) or WDR77 (S-WDR77) expression vector was cotransfected with the HF-CDK8 (A) or HF-CDK19 (B) expression vector into HeLa S3 cells. Expressed S-proteins were precipitated using anti-S-tag agarose and subjected to Western blotting. Lanes 1–4, 1% input of each cell lysate; lanes 5–8, S-tag pulldown fraction of each cell lysate. Arrowheads indicate each tagged protein. C, bacterially expressed GST-CDK8 or GST-CDK19 was immobilized on glutathione-Sepharose gel and incubated with each purified S-protein. After extensive washing, the retained proteins were detected by Western blotting. Lane 1, 2% input of S-PRMT5 or S-WDR77; lane 2, S-proteins bound to GST alone; lane 3, S-proteins bound to GST-CDK8; and lane 4, S-proteins bound to GST-CDK19. D, in vitro methyltransferase assay of anti-FLAG purified HF-CDK8 or HF-CDK19 complex. The purified complex was incubated with four purified bacterially expressed recombinant histone proteins in the presence of S-adenosyl-l-methyl-[3H]methionine. Fluorography (upper panel) and Coomassie Brilliant Blue (CBB)-stained histone proteins (lower panel) are shown. Lane 1, mock-purified fraction; lane 2, HF-CDK8-containing Mediator fraction; lane 3, HF-CDK19-containing Mediator fraction. E, in vitro methyltransferase assay using S-adenosylmethionine. Methylated histones were detected using anti-H4R3me2s and anti-histone H4 specific antibodies. Lanes 1–3 are as in D.