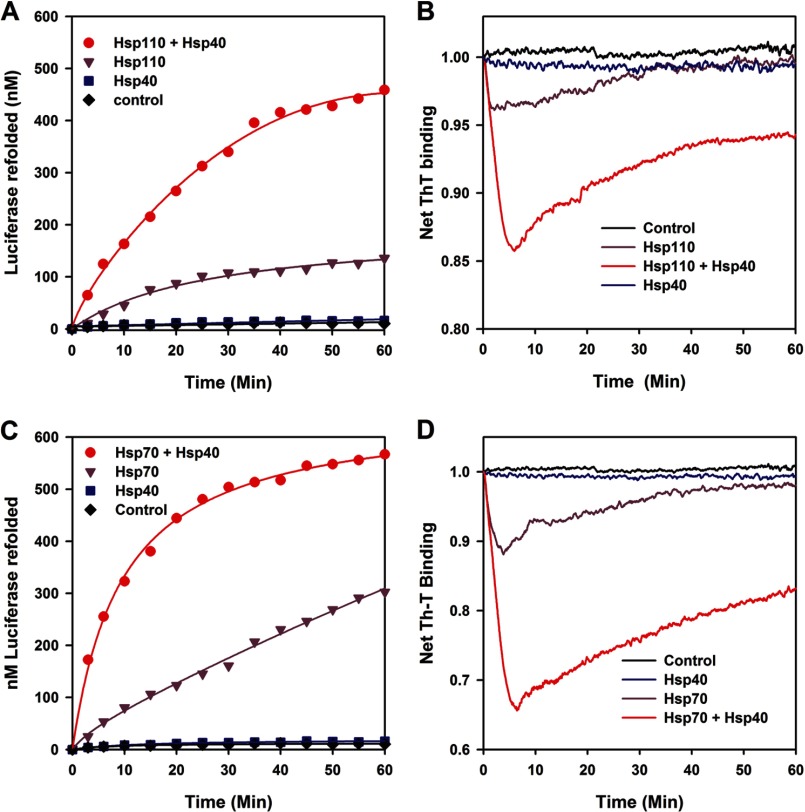
FIGURE 2.
Hsp110 (HSPH1) is an Hsp40- and ATP-dependent polypeptide unfolding/refolding chaperone similar to Hsp70. A and B, the activity (A) and the net ThT fluorescence (B) of stable misfolded FT luciferase (1 μm) were measured online at 22 °C in the presence of ThT (60 μm) at various time points as indicated, in the absence (black) or presence of ATP (5 mm) with Hsp110 alone (1 μm, purple), Hsp40 (DNAJA1) alone (0.5 μm, blue), or both Hsp110 + Hsp40 (red). C and D, the activity (C) and the net ThT fluorescence (D) of stable misfolded FT luciferase (1 μm) measured online without (black) or with ATP as in A and B, in the presence of Hsp70 alone (1 μm, purple), Hsp40 alone (0.5 μm, blue), or both Hsp70 and Hsp40 (red).