Fig. 1.
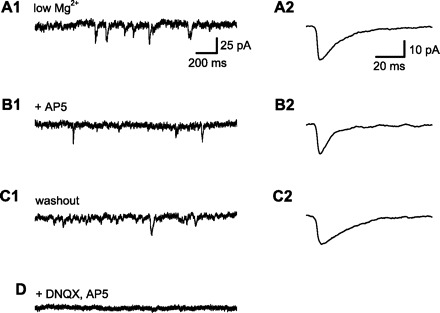
Measurement of N-methyl-d-aspartate receptor (NMDAR)-dependent component of spontaneous synaptic current. All data in this figure are from a single CA1 pyramidal neuron, clamped near the normal resting membrane potential (−70 mV). Spontaneous excitatory postsynaptic currents (sEPSCs) were recorded with low extracellular Mg2+ (50 μM) to allow NMDAR current flow at the negative holding potential and with GABA receptors blocked by a combination of extracellular bicuculline (10 μM) and intracellular Cs+. A1: 2-s sample of membrane current. sEPSCs were identified using an automated method and verified by visual inspection. A2: averaged sEPSCs from a 5-min baseline period. sEPSC amplitude, rise time (10–90%), and duration at half amplitude (half-width) were measured . B1: membrane current after application of d(−)-2-amino-5-phosphonopentanoic acid (d-AP5; 50 μM) to block NMDARs. B2: averaged sEPSCs from a 5-min period after wash-in of d-AP5. Note the decrease in EPSC half-width (from 14.7 to 6.9 ms) with little change in sEPSC amplitude, consistent with the selective block of slower NDMAR-mediated synaptic currents by d-AP5. C1: washout of d-AP5 restored sEPSCs to baseline. C2: averaged sEPSC after d-AP5 washout. Half-width (19.7 ms) was similar to baseline, indicating recovery of NMDAR-mediated synaptic current. D: application of both 6,7-dinitroquinoxaline-2,3(1H, 4H)-dione (DNQX) and d-AP5 abolished all synaptic currents.
