Fig. 5.
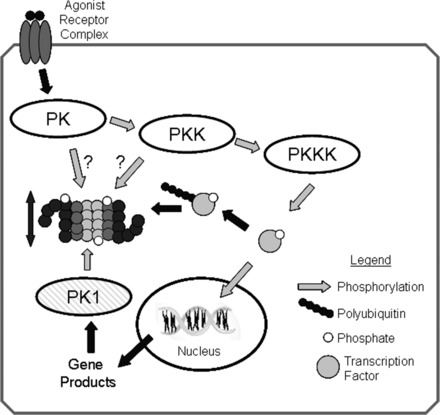
Regulation of proteasome activity by kinase cascades. In this scenario, binding of an agonist with a cell surface receptor initiates a phosphorylation cascade with an end effector protein kinase kinase kinase (PKKK) phosphorylating some transcription factor that can then enter the nucleus and interact with DNA resulting in gene transcription. Phosphorylation of signaling intermediates, in this case the transcription factor, is often a signal for ubiquitination and targeting to the 26S-proteasome for degradation. To amplify or dampen the effect of the signaling intermediate, any one of the protein kinases (PKs) within in the cascade might phosphorylate proteasome subunits, resulting in increased or decreased peptidase activity that would lessen or enhance availability of the signaling molecule accordingly. Another possibility would be for the gene product to activate a different protein kinase (PK1), which would phosphorylate proteasome resulting in activation and thus decreased availability of the transcription factor, essentially a form of feedback control. Reproduced from Powell (107) with the permission of Walters Kluwer Health.
