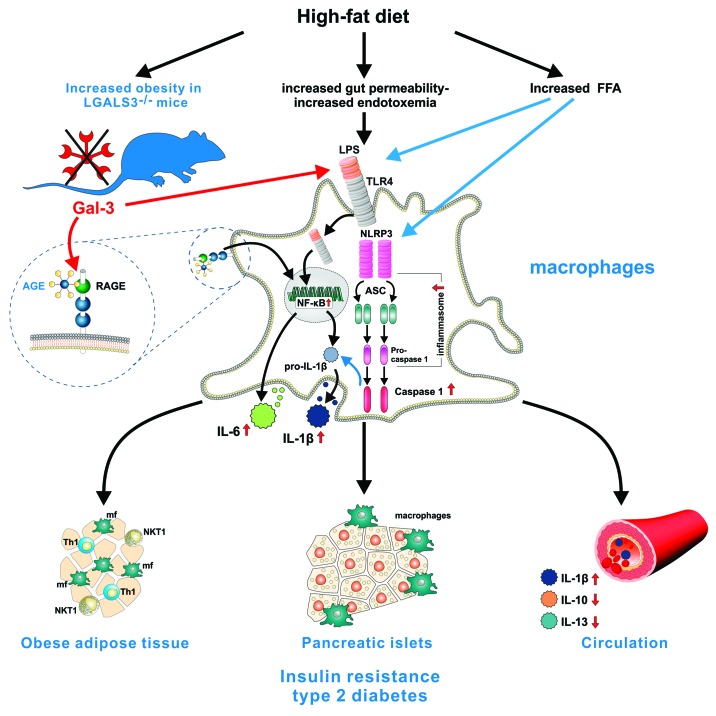
Figure 1. Gal-3 is an important regulator of adiposity, metaflammation, and type 2 diabetes. Fat-enriched diets increase plasma concentration of endotoxin (metabolic endotoxemia) and saturated fatty acids such as palmitate, that stress macrophages in metabolic tissues including adipose tissue and pancreatic islets leading to increased production of inflammatory cytokines IL-1β, TNF-α, IL-6, and chemokines which further attract immune cells into tissues and promote systemic inflammation. Gal-3 binds endotoxin and acts as a scavenger molecule for advanced glycation and lipoxidation end products. TLR4, the receptor for lipopolysaccharide (LPS), mediates the innate immune response to bacterial pathogens, but also triggers inflammatory processes upon exposure to certain dietary fats by activating NFκB pathway and NLRP3 inflammasome in macrophages leading to IL-1β secretion that causes insulin resistance. Ablation of Gal-3 leads to accelerated obesity and systemic inflammation, enhanced inflammation in adipose tissue and pancreatic islets, as indicated by increased type-1 T and NKT cells and pro-inflammatory macrophages in visceral adipose tissue and NLRP3 inflammasome and IL-1β expressing macrophages in islets in high-fat diet-fed mice. This is associated with the increased activation of NFκB signaling pathway through binding of AGE to upregulated receptor for AGE (RAGE) and activation of NLRP3 inflammasome by LPS and/or palmitate resulting in increased caspase-1-dependent IL-1β production and IL-6 by LGALS3−/− macrophages. The increase in circulating IL-1β and the decrease of anti-inflammatory IL-10 and IL-13 contribute to the development of insulin resistance and type 2 diabetes in Gal-3-deficient mice.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.