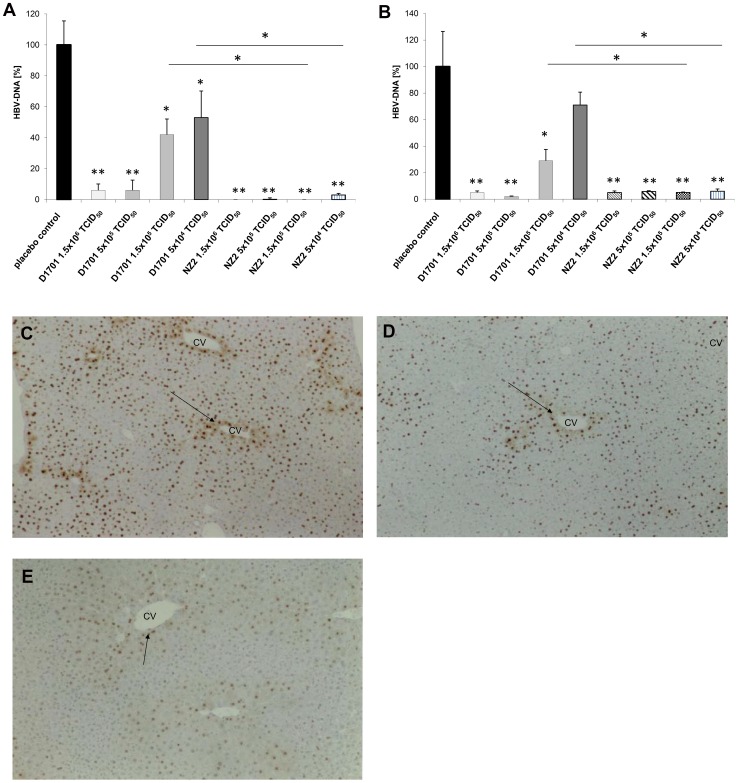
Figure 1. iORFV NZ2 is a more potent inhibitor of HBV than strain D1701 in HBV-transgenic mice.
iORFV D1701, iORFV NZ2 or placebo was administered i.p. every third day, three times in total, to HBV-transgenic mice expressing 107–108 HBV genome equivalents per ml plasma (n = 7 per group). HBV-specific DNA from plasma was analyzed by quantitative PCR and from livers by dot-blot hybridization as described previously [11], [18]. The figure shows means ± standard error of means (SEM) relative to placebo-treated animals where mean HBV-DNA content was set 100%. Treatment with iORFV reduced non-chromosomal HBV-DNA in the livers as compared to placebo animals (a) and in the plasma with the exception of the lowest dose of iORFV D1701 (b). iORFV NZ-2 was more potent in terms of its potential to inhibit HBV replication compared to strain D1701 with the two lower dosages resulting in significantly higher reduction of HBV-DNA (one-way ANOVA and post hoc analysis [Newman-Keuls Multiple Comparison Test] *p<0.05, **p<0.01). c) Immunohistological analysis of HBcAg expression in the livers of placebo-treated animals. Diffuse cytoplasmic staining in periportal areas [arrows, central veins (CV)] indicates viral capsids and ongoing HBV replication in placebo-treated mice. Cytoplasmic HBcAg as well as nuclear HBcAg-specific stain (for empty capsids) was strongly reduced in both iORFV (NZ2)-treated d) and iORFV (D1701)-treated mice e). Figures provide typical examples of the respective group of animals treated with a dose of 1.5×106 TCID50 iORFV NZ 2 or D1701, respectively.