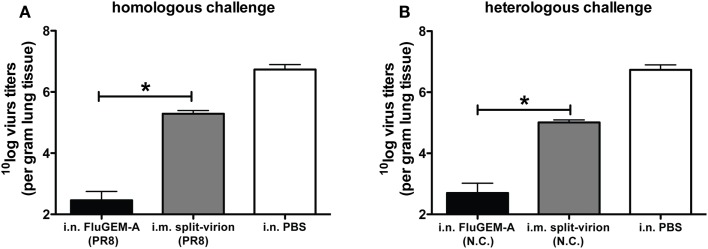
Figure 3.
Virus titers measured in the lungs of mice exposed to homologous and heterologous influenza challenge upon i.n. vaccination with FluGEM-A. Groups of six mice were vaccinated three times (day 0, 14, and 28) i.n. with FluGEM-A or i.m. with benchmark split-virion vaccine. Two groups were vaccinated with vaccine derived from PR8 strain (A), and two groups were vaccinated with vaccines derived from New Caledonia strain (B). One vaccination dose contained 5 μg HA and in addition to antigen, FluGEM-A vaccine contained 0.3 mg BLPs. Three weeks after the final immunization (day 49) mice were exposed to challenge with 100 TCID50 of PR8 virus. Lung virus titers were determined 5 days post-challenge. Virus titers measured after homologous challenge in the lungs of mice vaccinated i.n. with PR8-derived FluGEM-A vaccine were up to 100-fold lower compared to titers measured in lungs of mice vaccinated i.m. with PR8-derived split-virion vaccine (A). Virus titers measured after heterologous challenge in the lungs of mice vaccinated i.m. with New Caledonia-derived split-virion vaccine were significantly higher than titers measured in the lungs of mice vaccinated i.n. with New Caledonia-derived FluGEM-A vaccine (B). *p < 0.05; one-tailed Mann–Whitney U test (n = 6).