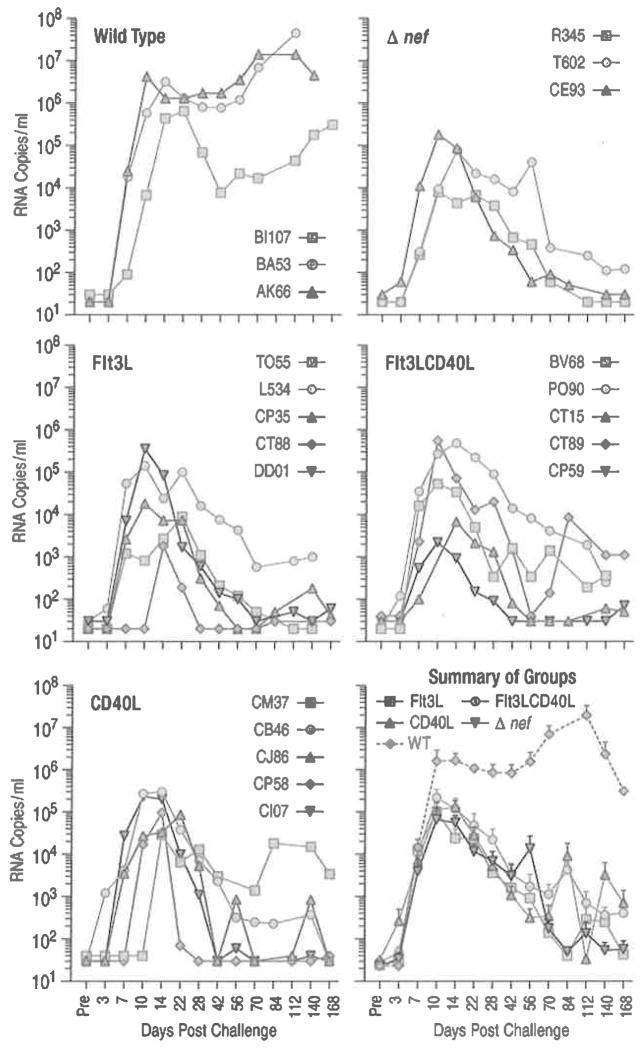
Fig. 6.4.
Increased number of circulating immature or partially matured DCs does not change the attenuated course of SIVmac239Δnef infection. Naive Indian RMs received Flt3L alone, Flt3L and CD40L, or CD40L alone (five animals per treatment group). Flt3L was administered for 7 days (100 μg/kg/day human Flt3L). To activate the cells, 0.6 mg/kg human CD40L trimer was given daily for 3 days after the Flt3L treatment. Another group of animals received only CD40L treatment. Animals were challenged with 2,000 TCID50 of SIVmac239Δnef across the palatine/lingual tonsils at the peak of DC mobilization 4 days post FLt3L treatment (Teleshova et al. 2004a) (or 1 day after CD40L). Control groups of untreated animals (three animals each) were challenged with 2,000 TCID50 of SIVmac239Δnef or wild-type SlVmac239 across the tonsils. Individual plasma viral loads (SIV RNA copies/ml of plasma) are shown for each animal in the different groups over time and the mean SIV RNA copies/ml (±SEM) are shown for each group in the summary plot. DC numbers in the blood were measured by four color flow cytometry. In the Flt3L-treated group the percentage of Lin−HLA-DR+ cells increased from 3.0%±0.5 to 33.1%±4.1 while in the Flt3LCD40L-treated group this number increased from 2.2% ±0.5 to 22.6%±6.2 (Mean±SEM). The percentage of Lin−HLA-DR+ cells in the CD40L-treated group did not change after treatment. The total number of Lin−HLA-DR+ (DC), Lin−HLA-DR+CD11c+CD123− (mDC), Lin−HLA-DR+CD11c−CD123+ (pDC), and Lin−HLA-DR+CD11c−CD123− (double negative) subsets increased by 16.3-, 1.8-, 3.8-, and 29.5-fold, respectively, for the Flt3L-treated group and by 12.3-, 2.8-, 3.1-, and 21.6-fold, respectively, for the Flt3LCD40L-treated group (each number being the fold increase (Post- divided by Pretreatment) of the average of the five animals within each group). The mobilized DCs were functional as evidenced by IL-12 production in response to CD40L stimulation in vitro, which was increased after Flt3L treatment as previously reported (Teleshova et al. 2004a). Of note, CD40L activation in vivo appeared only partially effective at best. In the CD40L-treated animals CD80 and CD86 expression increased by only 1.49- and 2.15-fold on mDCs and 1.34- and 1.98-fold on pDCs (respectively), while the levels of the FLt3L/CD40L-treated animals were basically comparable to those of the Flt3L-treated animals (other than CD86 on pDCs in the Flt3L-treated animals which increased by 1.6-fold). The limited effect of CD40L in the FLt3L-treated animals is likely due to the dose of CD40L being insufficient to activate the increased number of DCs following mobilization and/or the fact that the activated DCs migrated to the CD40L injection site (from the blood)