Figure 2.
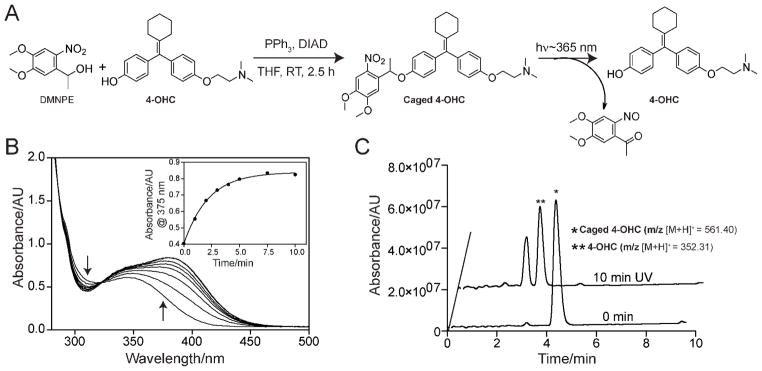
Synthesis of caged 4-OHC and photocleavage characterization. (A) Synthesis of caged 4-OHC under Mitsonobu reaction condition and activation (uncaging) of caged 4-OHC using 365 nm UV light. (B) Changes in UV-Vis absorbance during the photocleavage of caged 4-OHC in water:acetonitrile (1:1 v/v). Inset shows the change in absorbance at 375 nm over UV irradiation time. Note that the photocleavage is complete within 10 min of irradiation. (C) HPLC-MS chromatograms showing the quantitative formation 4-OHC from caged 4-OHC upon UV irradiation. Caged 4-OHC solutions, before (0 min) and after light exposure (10 min UV), were analyzed by HPLC-MS. Formation of 4-OHC was identified by the appearance of molecular mass corresponding to 4-OHC (m/z 352.31 [M+H]+). The peak other than the 4-OHC appeared in the 10 min UV chromatogram belongs to the cleaved caging group (see Figure 2A for the photocleavage reaction).
