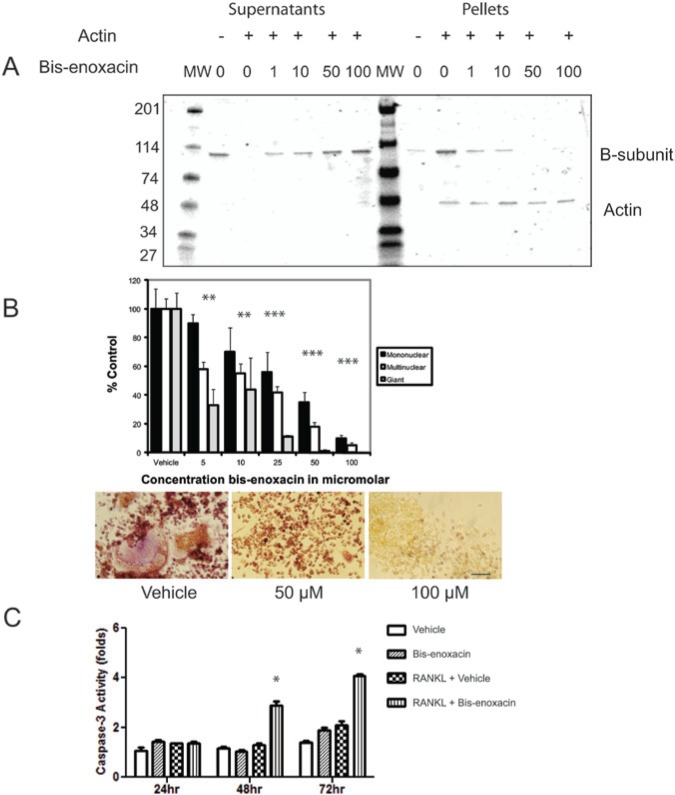
Figure 1.
BE inhibits binding between the V-ATPase B-subunit and microfilaments and osteoclast differentiation but stimulates caspase-3 activity in RANKL-treated RAW 264.7 cells. (A) The Recombinant Yeast B subunit (1 µM) was mixed with microfilaments (1 µM) together with vehicle or BE at the concentrations indicated. The B-subunit does not pellet during ultracentrifugation unless bound to microfilaments. The samples were subjected to ultracentrifugation, supernatants and pellets were collected, separated by SDS-PAGE, and stained with Coomassie Blue. BE dose-dependently shifted the B-subunit from pellet to supernatant, consistent with its blocking of the binding interaction. (B) RAW 264.7 cells were stimulated with RANKL and treated with vehicle or the concentrations of BE indicated. After 4 days in culture, cells were stained for TRAP activity, then counted by calibrated, blinded microscopists. The TRAP+ cells were grouped into mononuclear cells, multinuclear cells (2-10 nuclei), and giant cells (more than 10 nuclei). The control values were 114 giant cells, 299 multinuclear cells, and 980 mononuclear cells. BE reduced the number of multinuclear cells stained for TRAP activity (osteoclast-like cells) dose-dependently with an IC50 of approximately 10 µM. Asterisks indicate p < .05. The panels show representative photos from the experiment for vehicle or 50 and 100 µM BE. Scale bar equals 20 µm. (C) BE (50 µM) did not significantly alter caspase-3 activity levels in unstimulated RAW 264.7 cells but triggered a significant increase in caspase-3 activity in cells stimulated with RANKL. Asterisks indicate p < .05.