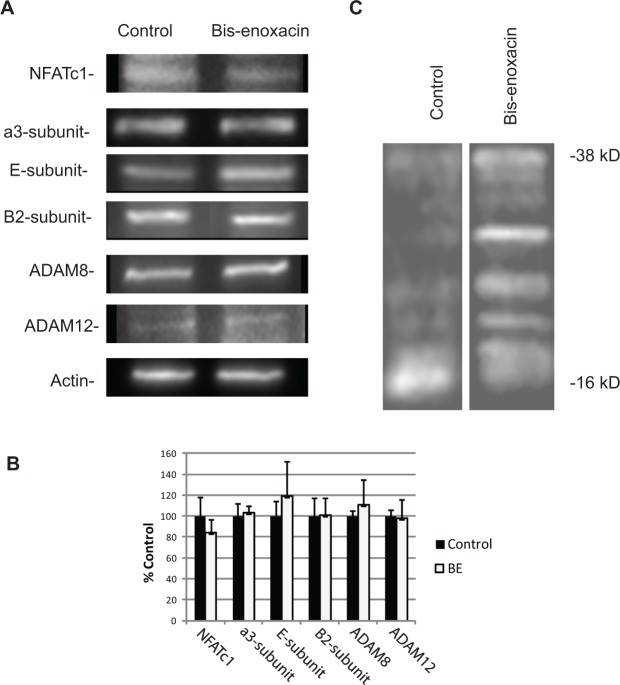
Figure 2.
Bis-enoxacin (BE) did not change the relative levels of osteoclast marker proteins, but altered proteolytic processing of TRAP5b. (A) Raw 264.7 cells were stimulated with RANKL plus vehicle or 50 µM BE. After 5 days, the cellular protein was extracted in SDS-PAGE sample buffer and spun at 100,000 x g to remove nucleic acids. The proteins were separated by SDS-PAGE, blotted, and probed with the antibodies indicated. An initial blot was performed to determine the loading that matched the amount of actin in the samples being compared. The blots displayed are samples from at least 3 trials. Anti-E, anti-B2, and anti-a3 subunit antibodies were used at a 1:500 dilution; anti-a disintegrin and metalloprotease domain ADAM8 and ADAM12 were used at a 1:250 dilution; and anti-nuclear factor of activated T-cells (NFATc1) was diluted 1:600. (B) BE did not alter the relative protein levels. Differences were not statistically significant by ANOVA. (C) Raw 264.7 cells were treated with RANKL plus vehicle, BE (50 µM), or enoxacin (50 µM). After 5 days, cells were harvested, and the cellular total proteins were separated by SDS-PAGE, blotted, and probed with anti-TRAP5B (1:500 dilution). Like enoxacin, treatment with BE altered the proteolytic activation of TRAP5b, indicated by the low amount of 16 kDa-activated TRAP and multiple higher-molecular-weight bands in the enoxacin-treated cultures compared with the control. Full-length TRAP5b pro-enzyme is 38 kDa.